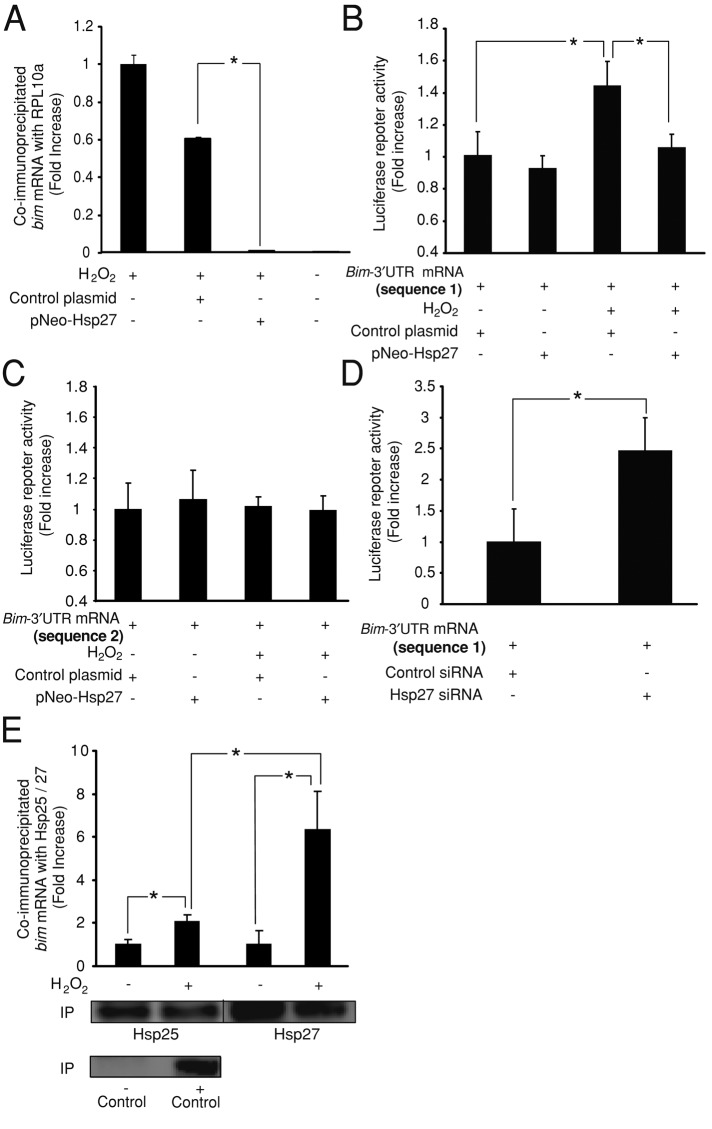
FIGURE 4:
Hsp27 regulates bim-3′UTR sequence during oxidative stress. (A) Quantification of bim mRNA levels bound to the ribosomal protein RPL10a present at the polysome. CGNs were treated with H2O2 (37.5 μM). Hsp27 overexpression reduced bim mRNA levels coimmunoprecipitated with RPL10a when compared with controls (*p < 0.05; n = 3). (B) CGNs were cotransfected with a luciferase reporter plasmid containing the bim-3′UTR (divided into two sequences, named 1 and 2) and either pNEO-Hsp27 or a control construct. Bim-3′UTR (sequence 1) neurons showed a significant increase in the luciferase activity after H2O2 (37.5 μM) addition (*p < 0.05; n = 3). Coexpression of pNEO-Hsp27 prevented this effect, significantly reducing the luciferase activity (*p < 0.05; n = 3). (C) Bim-3′UTR (sequence 2) neurons did not show any modification of the luciferase activity induced by H2O2 treatment or pNEO-Hsp27 coexpression. (D) CGNs were coelectroporated with the luciferase reporter plasmid containing the bim-3′UTR (sequence 1) and either Hsp25 siRNA or control siRNA. Hsp25 siRNA neurons displayed significantly higher luciferase activity than control siRNA neurons (*p < 0.05; n = 3). (E) Quantification of bim mRNA levels bound to the endogenous Hsp25 protein and to the wild-type form of the human Hsp27 (pNEO-Hsp27 construct) overexpressed in CGNs. H2O2 (37.5 μM) treatment significantly increased bim mRNA levels coimmunoprecipitated with the Hsp25/Hsp27 compared to control neurons (*p < 0.05; n = 4). The coimmunoprecipitated bim mRNA levels were significantly increased in H2O2-treated cells when Hsp27 was overexpressed (*p < 0.05; n = 4). As positive and negative controls for the immunoprecipitation, a specific antibody for Argonaute-2 protein and an unspecific serum were used, respectively. Argonaute-2 levels were detected by Western blot. bim mRNA levels were not detected in the negative control.