FIGURE 1:
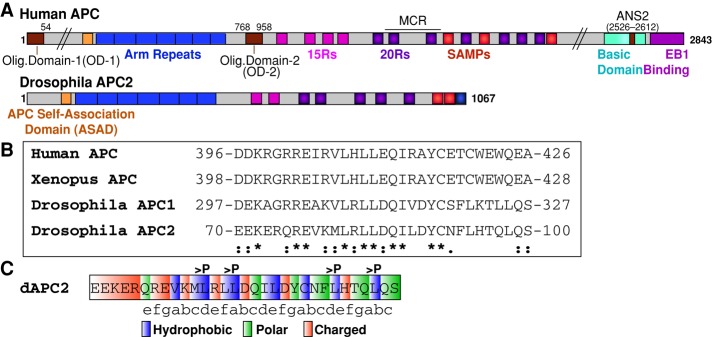
The ASAD is a conserved N-terminal coil. (A) Schematic representation of human APC and Drosophila APC2. ANS2, actin nucleation sequence 2; Arm repeats, Armadillo repeats (blue); ASAD, APC self-association domain (orange); MCR, mutation cluster region; 15Rs, 15–amino acid repeats (pink); 20Rs, 20–amino acid repeats (purple). (B) Sequence alignment of ASAD between human, Xenopus, and Drosophila APC proteins (*identical; [:], conserved substitution; [.], semiconserved substitution). (C) The ASAD coil fits into the classic heptad repeat (abcdefg) motif, where a and d are hydrophobic, e and g are charged, and b, c, and f tend to be polar amino acids (Gruber and Lupas, 2003). The four residues changed to proline in the APC2-ASADPro mutant are indicated.
