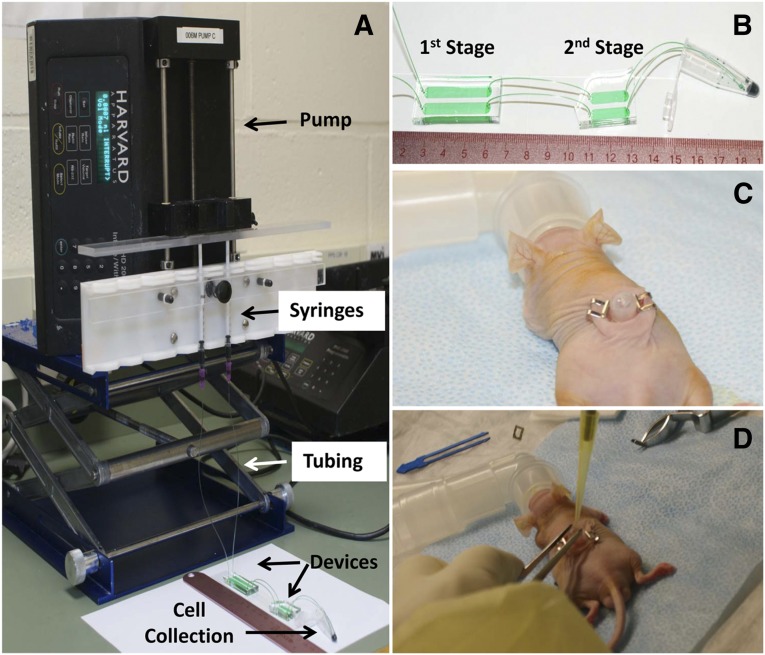
Figure 1.
Experimental setup of microfluidic cell separation and in vivo surgery. (A): The epidermal cell population was loaded in 1-ml syringes and flowed to devices via tubing. Flow rate was controlled using a pump, and released cells were collected in a 1.5-ml centrifuge tube. (B): A zoom-in view of the microfluidic device, which consists of two stages. The first stage of the device is to deplete CD71-positive nonstem cells from the epidermal cell population, and the second stage of the device is to enrich CD34-positive (CD34+) stem cells. The enriched stem cell population was collected in a 1.5-ml centrifuge tube. (C): The 6-mm-diameter full-thickness skin defects were created on the dorsal side of a Nu/Nu mouse. A silicone grafting chamber was inserted under the skin defect and secured by two Autoclips. (D): A mixture of microfluidic-enriched CD34+ stem cells, unenriched epidermal cells, and newborn dermal cells based on six different groups (Table 1) were injected into the graft sites of skin defect via the grafting chamber. Green food dye represents cell suspension and is only for the purpose of illustration.