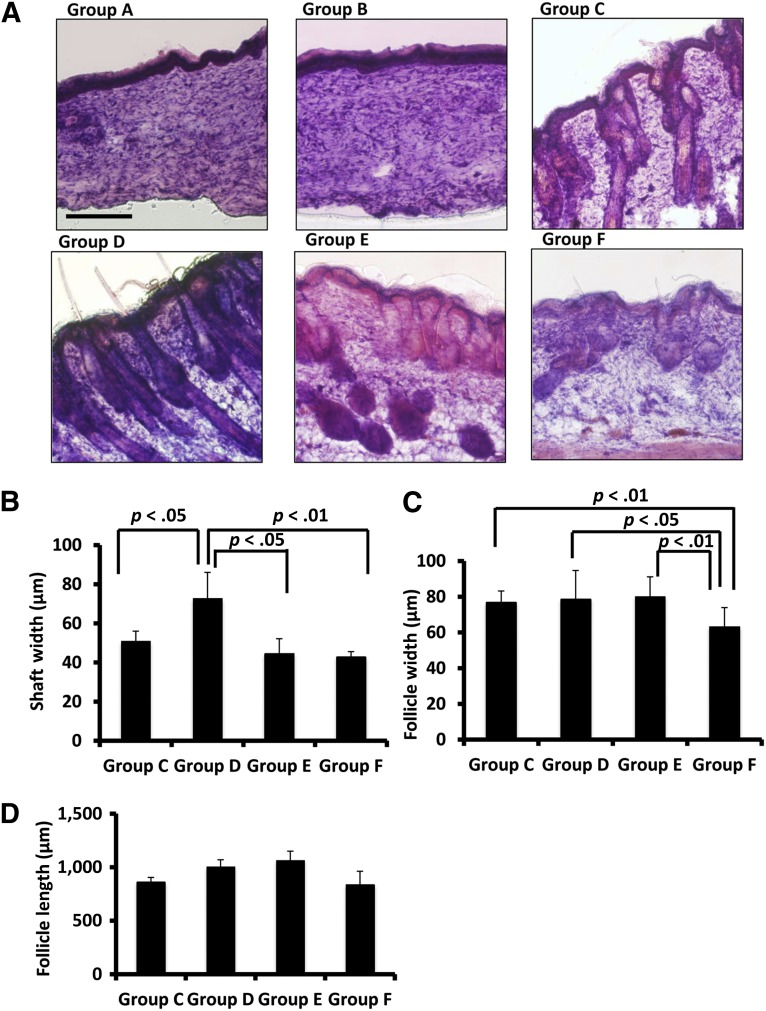
Figure 4.
Follicles and outgrown hair were observed in Nu/Nu mouse skin defect areas in groups C, D, and E. (A): Hematoxylin and eosin staining showed healing of skin defects in groups A and B. Groups C, D, and E had various degrees of follicle and hair formation in defect areas. Group F had follicles and hair shafts on the dorsal skin of the natural nude mice. (B): With the transplantation of both microfluidic-enriched CD34-positive (CD34+) cells and unenriched epidermal cells, group D had the widest hair shafts grow in defect areas (n = 6, mean ± SD; p < .05 versus group C; p < .05 versus group E; p < .01 versus group F). (C): Groups C, D, and E had similar width of hair follicles in skin grafts, but all of their hair follicles were wider than the natural skin of nude mice in group F (n = 8, mean ± SD; p < .01 groups C and E versus group F, respectively; p < .05 group D versus group F). (D): With both effects of microfluidic-enriched CD34+ cells and unenriched epidermal cell population, group D had longer hair follicles than groups C and F, respectively (n = 8, mean ± SD; p < .01). With microfluidic-enriched CD34+ cells and dermal fibroblasts, group E still had longer hair follicles than group C (with injection of only unenriched epidermal cells and dermal fibroblasts) (p < .01) and group F (the natural hair of nude mice) (n = 8, mean ± SD, p < .01, respectively). Scale bar = 250 μm (groups A–F).