Ex vivo cultured limbal epithelial transplantations are being widely practiced in the treatment of limbal stem cell deficiency. This report examined whether the limbal niche cells that nurture and regulate epithelial stem cells coexist in ex vivo limbal cultures and compared the inherent differences between explant and suspension culture systems in terms of spatial distribution of niche cells and their effect on epithelial stem cell proliferation, migration, and differentiation in vitro.
Keywords: Limbal stem cells, Limbal niche, Epithelial spirals, Self-organizing 3D niche
Abstract
Stem cells at the limbus mediate corneal epithelial regeneration and regulate normal tissue homeostasis. Ex vivo cultured limbal epithelial transplantations are being widely practiced in the treatment of limbal stem cell deficiency. In this report, we examined whether the limbal niche cells that nurture and regulate epithelial stem cells coexist in ex vivo limbal cultures. We also compared the inherent differences between explant and suspension culture systems in terms of spatial distribution of niche cells and their effect on epithelial stem cell proliferation, migration, and differentiation in vitro. We report that the stem cell content of both culture systems was similar, explaining the comparable clinical outcomes reported using these two methods. We also showed that the niche cells get expanded in culture and the nestin-positive cells migrate at the leading edges to direct epithelial cell migration in suspension cultures, whereas they are limited to the intact niche in explant cultures. We provide evidence that C/EBPδ-positive, p15-positive, and quiescent, label-retaining, early activated stem cells migrate at the leading edges to regulate epithelial cell proliferation in explant cultures, and this position effect is lost in early suspension cultures. However, in confluent suspension cultures, the stem cells and niche cells interact with each another, migrate in spiraling patterns, and self-organize to form three-dimensional niche-like compartments resembling the limbal crypts and thereby reestablish the position effect. These 3D-sphere clusters are enriched with nestin-, vimentin-, S100-, and p27-positive niche cells and p15-, p21-, p63α-, C/EBPδ-, ABCG2-, and Pax6-positive quiescent epithelial stem cells.
Introduction
The stratified squamous epithelial cell layer covering the corneal surface constantly undergoes regeneration by the activation and centripetal migration of stem cells from their niche at the corneal periphery, called the “limbus” [1–3]. In cases of unilateral limbal stem cell deficiency (LSCD), a small piece of limbal biopsy approximately 2 mm in size is harvested from the healthy fellow eye and used as the stem cell source for in vitro culture and expansion of limbal stem cells in autologous cultured limbal epithelial transplantations (CLETs) [4]. The two popular culture methods used for the CLET procedure are direct explant cultures (ECs) on complex biological substrates such as human amniotic membrane (hAM) [5–8] and the culture of enzymatically dissociated cell suspensions on mitotically inactivated murine NIH3T3 feeders [4, 9]. Both culture methods result in expansion of limbal stem cells in vitro and generate a sheet of epithelial monolayer that can be used to cover and regenerate the entire corneal surface of the affected eyes [10, 11].
The limbal region of the cornea not only acts as a physical boundary between the corneal and conjunctival epithelium but also serves as a barrier to block the progression of conjunctival vasculatures and lymphatics. Several studies based on in vivo examination of the limbal niche and palisades by immunohistochemistry have shown that it is a complex structure consisting of different cell types and unique extracellular matrix (ECM) components. Apart from epithelial stem cells, the limbal niche consists of different cell types, including neural crest-derived cell types such as melanocytes, mesenchymal-like stromal keratocytes, sensory neurons, vascular endothelial cells, and Langerhans cells for immune surveillance [12–19]. These niche cells nurture the stem cells and interact with and regulate them at the limbal boundary.
We often observe mildly pigmented cell clusters and mesenchymal-like stromal outgrowths in limbal explant cultures on hAM that are maintained beyond confluence. These stromal cells proliferate and migrate out of hAM and grow on the culture dish surface and are shown to be multipotent mesenchymal-like cells [20–23]. We also find similar spindle-shaped cells and pigmented cell clusters in limbal suspension cultures (SCs) on NIH3T3 feeders. Consequently, we explored whether the ex vivo limbal cultures have niche cells to nurture and regulate stem cells. In addition, the limbal niche and stem cells remain intact in ECs, whereas the tissue gets enzymatically digested and the cells are isolated as single cell suspensions for initiating SCs on NIH3T3 feeders. We hypothesized that there may be inherent differences between explant and suspension cultures in terms of the niche and stem cell interactions. In other words, we considered whether they are actually pure cultures of limbal epithelial stem cells or a mixed coculture consisting of the limbal niche cells, limbal stem cells, and differentiated mature epithelial cells. If they are mixed cultures, then we hoped to understand whether there will be any differences between the explant and suspension cultures in terms of spatial distribution of niche cells and their effect on epithelial stem cell proliferation, migration, and differentiation in vitro.
In this report, we provide evidence that both the explant and suspension cultures support the expansion of limbal epithelial stem cells as well as niche cells. It appears that even in the absence of an intact niche in vitro, when cultured together, the epithelial stem cells and niche cells carry an inherent internal program with which they interact with and regulate each other. They display spiraling cell migration patterns in vitro, similar to the centripetal movements seen on the corneal surface, enabling them to self-organize to form three-dimensional (3D) stem cell compartments resembling the limbal crypts.
Materials and Methods
The study was approved by the institutional review board of the L.V. Prasad Eye Institute, Hyderabad, India, and the research followed the tenets of the Declaration of Helsinki.
Establishment and Maintenance of Human Limbal Epithelial Cell Cultures
Corneoscleral rims of rejected corneas obtained from donors younger than 80 years were used for the study (n = 25). The tissues were collected from the Ramayamma International Eye Bank at the L.V. Prasad Eye Institute and were used within 48–72 hours after harvest. To establish explant cultures of limbal epithelium, the corneoscleral rims were gently scraped with a scalpel on the concave surface to remove the endothelial cells and rinsed three times with phosphate-buffered saline (PBS) containing double-strength antibiotics and fungizone. The rims were trimmed on either side by visualizing the palisades under a dissection microscope and then chopped into smaller pieces of approximately 1 mm and explanted onto either hAM (for fluorescence-activated cell sorting [FACS]) or serum-coated glass coverslips (for immunocytochemistry [ICC]) and incubated at 37°C for 30 minutes to allow for tissue adhesion. The cultures were maintained in human corneal epithelial (HCE) growth medium containing Dulbecco’s Modified Eagle’s Medium: Nutrient Mixture F-12 supplemented with 10% fetal bovine serum, 1× GlutaMAX, 1× penicillin-streptomycin, 10 ng/ml human recombinant epidermal growth factor, and 5 μg/ml human recombinant insulin (Invitrogen, Carlsbad, CA, http://www.invitrogen.com), with regular media changes on alternate days for up to 2 weeks.
To establish limbal suspension cultures, the processed limbal rims were chopped into four quarters and incubated in basal medium containing 1.2 U/ml dispase II and 0.3 mg/ml collagenase type IA (Sigma-Aldrich, St. Louis, MO, http://www.sigmaaldrich.com) for 1 hour at 37°C. The loosened epithelium was gently scraped and released. The residual stromal tissue was removed, and the epithelial cell suspension was pelleted and further digested with 0.25% trypsin/EDTA at 37°C for 5 minutes to prepare single-cell suspensions. The cell suspensions were passed through a 70-μm cell strainer (BD Biosciences, San Diego, CA, http://www.bdbiosciences.com), spun down to collect the cell pellet, and washed once with basal medium. The final cell pellet was suspended in HCE medium, plated on to mitomycin-inactivated NIH3T3 feeders, and cultured for approximately 1–2 weeks before processing for either ICC or FACS analysis.
BrdU Pulse Labeling and Long-Term Chase
To label actively dividing cells, the cultures on glass coverslips are fed with 5-bromo-2′-deoxyuridine (BrdU) containing growth medium (100 μM/mL) for 30 minutes (pulsing) and then washed with PBS before fixing them for ICC. To detect slow-cycling and early activated stem cells, the cultures are pulsed with BrdU for 1 hour, washed with PBS, and cultured for another 10 days (chasing) in growth medium before fixing them for ICC. For BrdU label detection, the fixed cells are treated with denaturation buffer containing 2N HCl, 0.5% Triton X-100, and 0.5% Tween 20 for 30 minutes at room temperature and neutralized immediately with freshly prepared 1 mg/ml sodium borohydride solution. The cells are washed three times with PBS, blocked with 10% serum, and processed for immunostaining using anti-BrdU antibody.
Immunocytochemistry and Confocal Imaging
The cells grown on glass coverslips are fixed with 3.5% formaldehyde in PBS and permeabilized with 0.5% Triton X-100 in PBS for 10 minutes each, followed by three PBS washes. The permeabilization step was skipped for SSEA4 staining. The cells are blocked with 10% serum in PBS at room temperature for 1 hour and then sequentially incubated with specific primary and fluorescent dye conjugated secondary antibodies at appropriate dilutions (supplemental online Table 1) at room temperature for 1 hour each, with three PBS washes in between. Propidium iodide (PI) or DAPI (4′,6-diamidino-2-phenylindole) were used as counterstains. The cells were then washed and mounted on glass slides and imaged using an epifluorescence microscope (Olympus IX71; Olympus, Tokyo, Japan, http://www.olympus-global.com) or a confocal microscope (LSM 510; Carl Zeiss, Jena, Germany, http://www.zeiss.com). The images were analyzed using Image Pro Express (Media Cybernetics, Bethesda, MD, http://www.mediacy.com) and LSM 510 Meta, version 3.2 (Carl Zeiss), respectively, and the composites were prepared using Adobe Photoshop CS (Adobe Systems Inc., San Jose, CA, http://www.adobe.com).
Quantification of Cells by FACS Analysis
The limbal cultures were harvested by trypsin treatment to prepare single-cell suspensions and further fixed and processed for immunostaining, as described above. Cells stained with secondary/isogenic antibody were used as negative controls. The samples were then analyzed using a FACS Aria I cell sorter, and the analysis was performed using FACS Diva software (BD Biosciences). Percentage of positivity was represented as mean percentage of positives ± SD of three experimental replicates. The stained cell preparation was also mounted and imaged for confirmation of signal specificity (supplemental online Fig. 1).
Results
Expression of Epithelial and Stem Cell Markers in Limbal Cultures
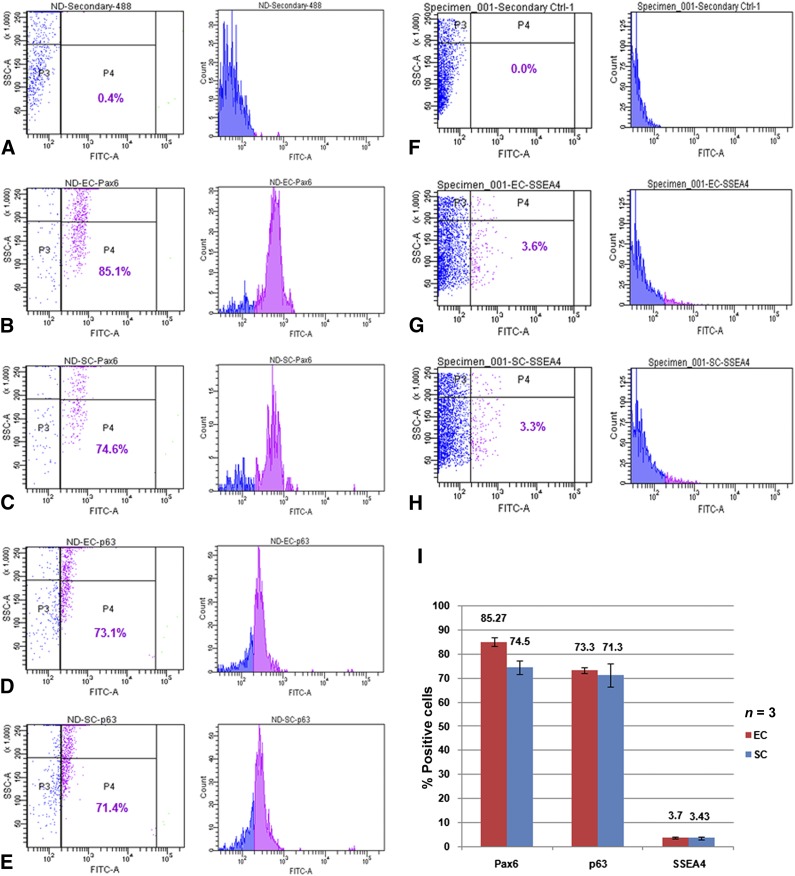
To examine the differences in stem cell content between limbal ECs on hAM and SCs on NIH3T3 feeders, we established parallel cultures using the same donor limbal rims and examined the stem cell content of the cultures by FACS analysis using antibodies against antigens such as Pax6, p63, and SSEA4. As shown in Figure 1, both ECs and SCs resulted in comparable levels of Pax6 (85.1% and 74.6%, respectively, of total cells analyzed), p63 (73.1% and 71.4%, respectively, of total cells analyzed), and SSEA4 expression (3.6% and 3.3%, respectively, of total cells analyzed). Figure 1I summarizes the mean percentage of positive cells expressing different antigens in three independent experiments. It was not surprising to observe a high percentage of p63-positive cells in our cultures because the primary antibody we used could recognize all the six p63 isoforms (supplemental online Table 1), which are expressed not only by stem cells but also by transiently amplifying cells that constitutes the major pool of p63-positive cells in culture. However, a small subset of 3%–4% of cultured limbal cells expressed the pluripotent/early progenitor marker SSEA4. This finding confirmed an earlier report that quantified ∆Np63α-expressing stem cells in suspension cultures [10] and suggested no significant difference between the two popular culture systems in terms of stem cell content and expansion efficiency.
Figure 1.
Fluorescence-activated cell sorting (FACS) characterization of cultured human limbal epithelial cells. Top panels (A, F) show the event profiles for secondary antibody controls; the gates were set to exclude all cells showing background fluorescence. (B–E, G, H): FACS profile of Pax6 (B, C), p63 (D, E), and SSEA4 (G, H) expression in limbal cells cultured under EC (B, D, G) and SC (C, E, H) methods. (I): Bar graph representing mean percentage of positive cells for different markers in EC and SC. Error bars represent standard deviations of three independent experimental values. Abbreviations: EC, explant culture; FITC, fluorescein isothiocyanate; SC, suspension culture; SSC, side scatter.
Spatial Distribution of Limbal Niche Cells In Vitro
We assessed whether our limbal cultures had any niche cells. Limbal stromal cultures are known to contain nestin-positive cells [24, 25], which may be neural, mesenchymal, or neural-crest-derived cell types that are part of the native limbal niche. We examined our cultures for the presence and localization of nestin-positive cells using ICC because it not only allows us to test for the presence or absence of antigen expression (percentage of positivity) but also provides evidence of subcellular localization (nuclear, cytosolic, and pan expression) and relative positioning of individual cells within cultures (explant vs. leading edges of epithelial outgrowths).
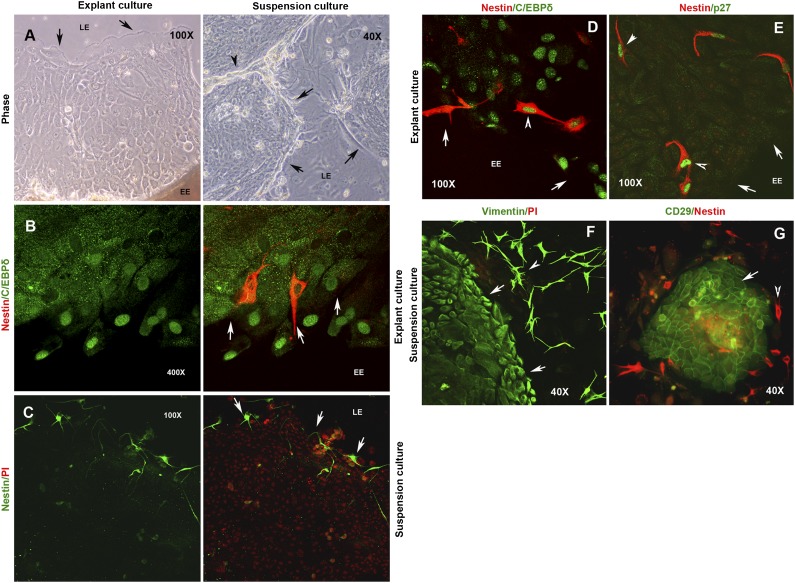
Limbal cultures were established using both EC and SC methods on glass coverslips with or without feeders (Fig. 2A) and subsequently processed for ICC using anti-nestin antibody. Although we observed nestin-positive spindle-shaped cells in both cultures, it was surprising to find them distinctly localized in each of the culture methods. In explant cultures, these cells were restricted to the explant edge (Fig. 2B), and none were detected in the middle or at the leading edges of growth zones (data not shown). In contrast, they were localized at the leading edges or the periphery of growing holoclones in suspension cultures (Fig. 2c). We also found a subset of rare nestin-positive cells coexpressing the limbal epithelial stem cell marker C/EBPδ (Fig. 2D). Moreover, in both culture systems, the nestin-positive cells exclusively expressed the Cip/Kip family of cyclin-dependent kinase inhibitor (CKI) p27 in the nucleus (Fig. 2E). p27 is a reversible cell-cycle arrest marker known to be expressed by quiescent niche and stem cells, and its nuclear localization is suggestive of mitotic arrest.
Figure 2.
Differential localization of niche cells in explant culture (EC) and suspension culture (SC) systems. (A): Phase image of growing cultures established by EC and SC methods. (B): Localization of nestin-positive cells (red) at the EE in the EC system. C/EBPδ expression is shown in green. (C): Localization of nestin-positive cells (green) at the LE in the SC system. Expression of stromal cell markers in EC and SC systems. (D): Expression of C/EBPδ (green) in a rare subset of nestin-positive cells (red) at the EE in an EC system. (E): Coexpression of p27 (green) in all nestin-positive cells (red). (f) Expression of vimentin (green) both by the epithelial cells and the stromal cells migrating at the LE in an SC system. (G): Exclusive expression of CD29 (green) by the limbal epithelial cells, whereas the nestin-positive cells (red) are seen migrating at the colony periphery. The cells were counterstained with propidium iodide (PI) to label the nuclei in red. Arrows indicate the EE and LE boundaries. Arrowheads mark the cells expressing different antigens. Abbreviations: EC, explant culture; EE, explant edge; LE, leading edge; SC, suspension culture.
We next examined the expression of another cytoskeletal protein, vimentin, which is known to be expressed by the limbal-basal epithelial cells and stromal cells. The entire cell sheet formed by the explant outgrowths and the growing holoclones stained positive for vimentin. Interestingly, we also observed intense vimentin-positive spindle-shaped cells migrating at the leading edges of growing holoclones in suspension cultures (Fig. 2F). Earlier studies, based on FACS analysis, reported CD29 as a marker of mesenchymal-like cells of the anterior limbal stroma [22, 23, 26]. However, we observed that the epithelial cells of individual holoclones specifically expressed CD29, and the colony periphery was lined by nestin-positive cells (Fig. 2G), emphasizing the uniqueness and usefulness of ICC in the clear identification of cell types expressing different antigens. It is important to note that NIH3T3 feeders are negative for nestin and CD29 expression and show only weak positivity for vimentin. These observations suggest that niche cells are also expanded in limbal cultures, regardless of the culture method used; however, their localization within the cultures is different, suggesting distinct mechanisms of stem cell-niche interactions in vitro.
Spatial Distribution of Cells Expressing Different Cell Cycle Regulators
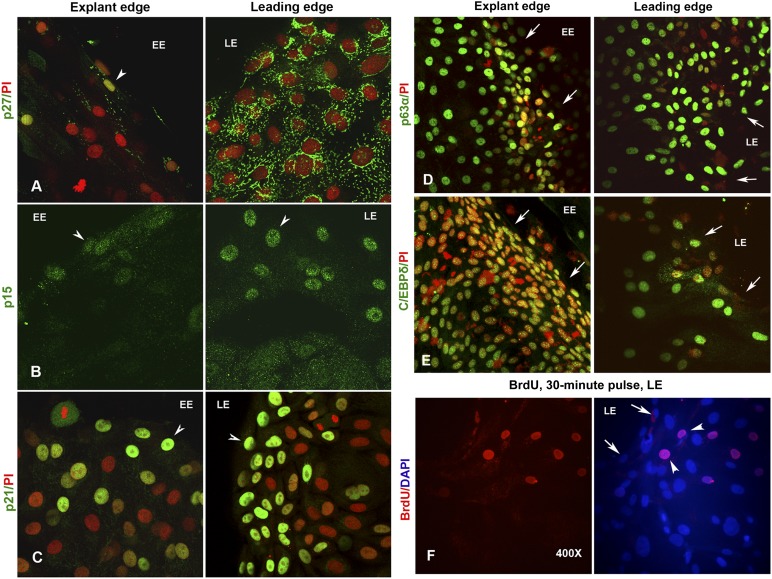
As discussed in the previous section, nuclear expression of the reversible arrest marker p27 was noted exclusively in nestin-positive cells at explant edges. Although the epithelial cells at the explant edge had no (or weak) cytosolic signals for p27, the leading edge cells showed bright and punctate cytosolic staining that suggests stable accumulation of inactive proteins (Fig. 3A). This led us to examine the expression patterns of two other CKIs, p21 (belonging to the Cip/Kip family of CKIs, a terminal arrest and differentiation marker) and p15 (belonging to the INK family of CKIs) and their roles in regulating limbal epithelial cell cycle, proliferation, and differentiation.
Figure 3.
Expression of cyclin-dependent kinase inhibitors in limbal explant cultures. (A): Nuclear expression of p27 (green) in few cells at the EE (left). Note the complete absence of fluorescence signal in the surrounding epithelial cells marked by PI (red). However, the epithelial cells at the LE show intense cytosolic signal for p27 (right). (B): Nuclear expression of p15 (green) only in few cells at the EE (left) and at the LE (right). Only background cytosolic fluorescence was noted in the remaining epithelial cells of the middle zone. (C): Bright nuclear expression of p21 (green) in cells localized to both the EE (left) and LE (right) cells. Expression of stem cell markers in limbal explant cultures. Epithelial cells expressing p63α (D) and C/EBPδ (E) at the EE (left) and LE (right) of explant cultures. The cells were counterstained with PI to label the nuclei in red. (F): Proliferating epithelial cells that incorporated the BrdU label (red) in a 30-minute pulse. Note the localization of BrdU-positive cells at approximately two or three cells behind the migrating or leading edge. The cells were counterstained either with PI (red) or DAPI (blue) to label the nuclei. Arrows indicate the EE and LE boundaries. Arrowheads mark the cells expressing different antigens. Magnification, ×400 for all images. Abbreviations: BrdU, 5-bromo-2′-deoxyuridine; DAPI, 4′,6-diamidino-2-phenylindole; EE, explant edge; LE, leading edge; PI, propidium iodide.
On immunostaining of explant cultures, we found a majority of cells expressing varying levels of p21 in the nucleus, suggesting that the cultures are dominated by transiently amplifying and differentiating cells. In ECs, intense p21-positive cells were present at all locations: explant edge, leading edge, and in the middle of the epithelial sheet. However, a zone of two or three cells at both the leading edge and the explant edge distinctly expressed very high levels of p21 in the nucleus, suggesting their arrested state (Fig. 3C). Interestingly, we observed exclusive expression of nuclear p15 in a zone of approximately one or two cells at the explant edge and approximately four or five cells at the leading edge, whereas the entire cell sheet in between showed only background staining in the cytosol (Fig. 3B). This clearly suggests that a subpopulation of cells at both the explant edge and the leading edge are nondividing. However, a majority of cells within holoclones expressed high levels of p21 and low levels of p15 in the nucleus, suggesting that the directional cues may be missing in early stage suspension cultures (supplemental online Fig. 2).
Identity of Quiescent Cells at the Leading Edge of Explant Cultures
In order to assess the identity of quiescent cells at the leading and explants edges, we examined the expression of the stem cell markers p63α and C/EBPδ and found that they expressed both markers at high levels in their nuclei (Fig. 3D, 3E). To confirm the proliferative status of these cells, we pulsed them with BrdU (100 μM) for 30 minutes and processed for ICC. BrdU-positive epithelial cells were found to be trailing behind a zone of four or five cells at the leading edge (Fig. 3F). This was also confirmed by Ki67 staining (data not shown). Cells at the leading edge appear to be nondividing and could be either quiescent stem cells or terminally differentiated cells.
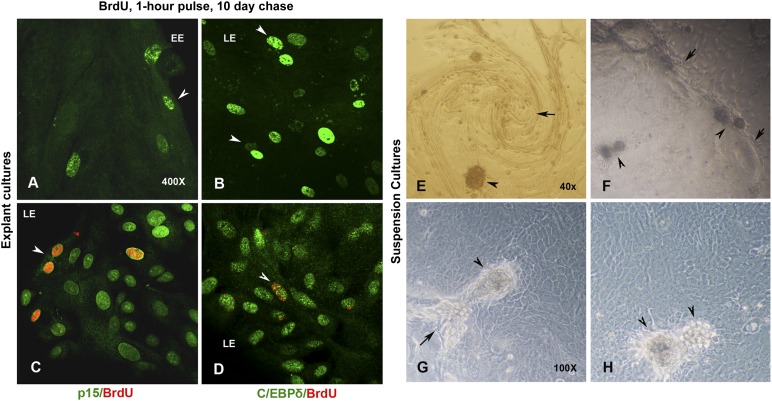
We pulsed an early stage culture (in which the epithelial outgrowths had just started from explants) with BrdU (100 μM) for 1 hour, chased for 10 days, and fixed the confluent cultures for ICC. As shown in Figures 4A and 4B, slow-cycling and label-retaining cells were found at the explant and leading edges, respectively. Interestingly, the label-retaining cells showing bright and uniform nuclear staining for anti-BrdU were found in greater numbers at the leading edges. We hypothesized that the label-retaining cells could be early activated stem cells that stopped dividing within one or two cycles of cell division or transiently amplifying (TA) cells that underwent terminal differentiation after the incorporation of the BrdU label. Hence, we examined the BrdU-label-retaining cells for the coexpression of p15, p21, C/EBPδ, and K12 antigens. Interestingly, some of the p15-positive leading edge cells retained the label and coexpressed the stem cell marker C/EBPδ (Fig. 4C, 4D). Although some of the label-retaining cells also coexpressed p21, we did not observe a single K12-positive cell that retained the BrdU label at the end of a 10-day chase (supplemental online Fig. 3A, 3B). These observations suggest that, apart from the native niche (explant edge), a subset of early activated stem cells migrate at the leading edge and remain quiescent as label-retaining BrdU- , p15-, or CEBPδ-positive cells, whereas another subset continues to proliferate, lose the label after several cell division cycles, and terminally differentiate to become BrdU-negative, K12-positive mature epithelial cells.
Figure 4.
Localization of slow-cycling stem cells in limbal explant cultures. (A): Epithelial cells that retained BrdU (green) after a 1-hour pulse and a 10-day chase. Note the localization of BrdU-positive cells at both the EE and the LE of explant cultures. (B): Note the intense and uniformly labeled slow-cycling cells that are prominent at the LE. (C): BrdU label (red) retaining cells at the LE coexpressing p15 (green). (D): BrdU label (red) retaining cells at the LE coexpressing C/EBPδ (green). Magnification, ×400 (A–D). Centripetal migration of cells and self-organizing three-dimensional (3D) niche compartments in limbal cultures. (E): Suspension cultures of limbal epithelial cells migrating in whirling pattern. Note the emerging 3D-sphere clusters within the spirals. (F): 3D-sphere clusters emerging at the migrating edges or LEs and within the growing epithelial holoclones. Detailed view of 3D cell clusters at the junction of two colonies (G) and at the middle of a holoclone (H). Note the melanin pigmentation within these cell clusters. Arrows mark the clonal boundaries and junctions. Arrowheads indicate the 3D cell clusters. Magnification, ×40 (E, F), ×100 (G, H). Abbreviations: BrdU, 5-bromo-2′-deoxyuridine; EE, explant edge; LE, leading edge.
Self-Organizing Niche-Like 3D Compartments in Adherent Cultures of Suspended Cells
The mesenchymal-like cells of the limbal stroma are known to be multipotent, similar to bone marrow-derived cells. Some reports have also shown that suspension cultures of limbal stromal cells, under serum-free conditions, could generate spheres containing cells with neural potential and also very early progenitors expressing Oct4, Sox2, and Nanog [22, 25, 27–29]. As shown in Figure 4B–4D, we observed self-organizing 3D spheres in mid- to late-stage suspension cultures, specifically originating at the leading edges or at junctions of adjacent epithelial holoclones that merge with each other. Occasionally, we also observed spiraling or whirling patterns of cell migration similar to those created by the centripetal migration of limbal cells at the corneal center of GFP/β-gal mosaic mice [30–32]. Self-organizing 3D spheres were also formed within such epithelial spirals (Fig. 4A). Because the spheres were found predominantly at leading edges and at clonal junctions and are mildly pigmented, it became interesting to explore the nature of these compartments and the identity of cells within these 3D cell clusters.
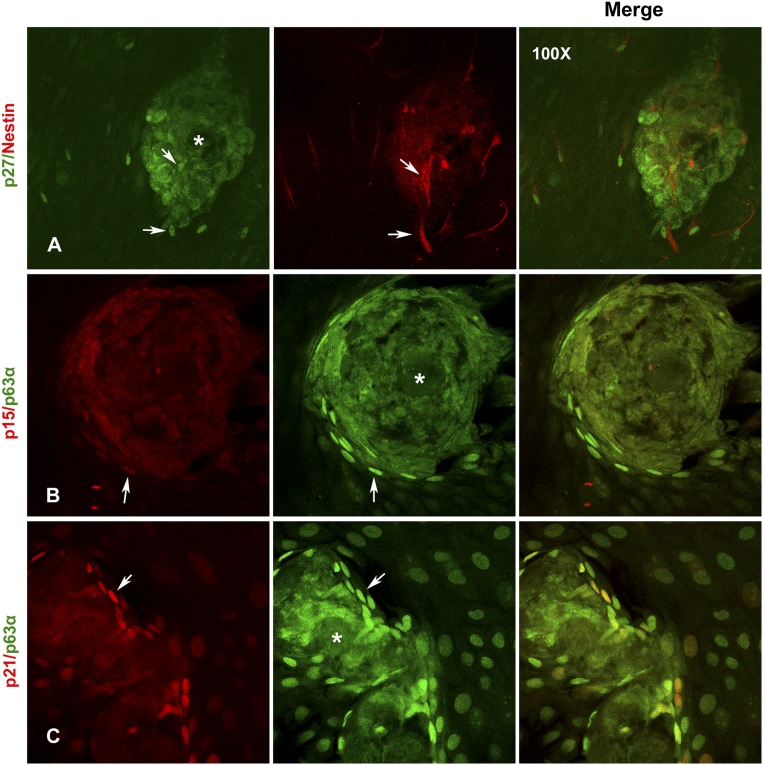
In confocal Z sections, the cells within the spheres are seen to be arranged in whirling patterns, with larger nuclei containing highly heterochromatinized DNA, and are brightly stained with PI (supplemental online Fig. 4A–4D). Some spheres also had cell-free central core possibly made of ECM proteins and pigments that appear as signal-free hollow (asterisk in Fig. 5). Interestingly, nestin-positive cells are seen both within and surrounding these sphere clusters and are positive for nuclear p27 (Fig. 5A). The epithelial cells immediately surrounding the spheres expressed high levels of p15, p21, and p63α and are brightly stained when compared with the surrounding TA cells, suggesting that they are quiescent stem cells (Fig. 5B, 5C).
Figure 5.
Localization of quiescent stem cells in close proximity to the niche-like three-dimensional (3D) compartments. (A): Localization of p27 (green) and nestin (red) coexpressing niche cells within and around the 3D-sphere clusters. Note the cytosolic expression of p27 in the cells within the spheres. (B): Localization of p15 (red) and p63α (green) coexpressing limbal epithelial stem cells around the 3D-sphere clusters. ∗, Note the cell-free central core in this sphere with possible extracellular matrix components and melanin pigmentation that blocks the fluorescence signal and appear as a hollow in the center. (C): Localization of p21 (red) and p63α (green) coexpressing limbal epithelial stem cells around the 3D-sphere clusters. All images are at ×100 magnification.
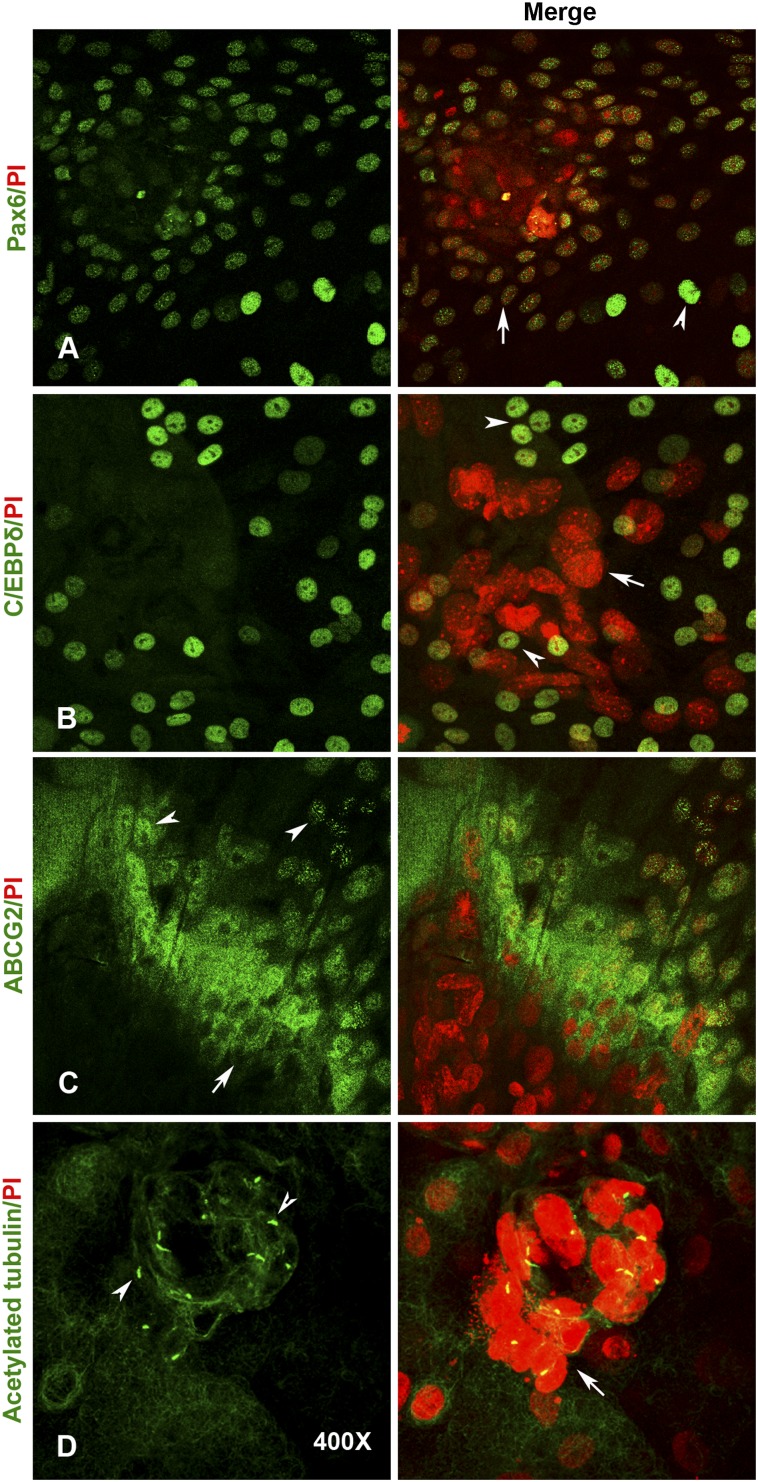
We checked for the expression of other stem cell and corneal markers such as ABCG2, C/EBPδ, and Pax6. Epithelial cells immediately surrounding the spheres distinctly expressed ABCG2 on the membrane. A few cells away from the spheres also showed punctate nuclear staining for ABCG2 (Fig. 6C). It remains to be understood whether this nuclear localization has any functional significance in terms of stem cell activation and initiation of differentiation. We also observed intense C/EBPδ-expressing cells both within and surrounding the 3D cell clusters (Fig. 6B). Interestingly, some of the cells with large and heterochromatinized nuclei within the 3D spheres and those immediately around expressed Pax6, confirming their corneal or ocular origin. The levels of Pax6 expression was low in these cells when compared with the bright staining of a few differentiated epithelial cells away from these clusters (Fig. 6A).
Figure 6.
Expression of limbal stem cell markers within three-dimensional-sphere (3D-sphere) clusters. (A): Expression of Pax6 (green) in cells within the sphere cluster. Note the weak expression (arrow) when compared with the intense staining observed in differentiating cells (arrowhead) away from the 3D cluster. (B): Expression of C/EBPδ (green) in cells surrounding the sphere cluster. (C): Exclusive membrane localization of ABCG2 antigen (green) in epithelial stem cells adjacent to the 3D cluster (arrow). Note the distinct nuclear expression of ABCG2 in only few cells that are separate from the spheres (arrowhead). (D): Exclusive expression of primary cilium (green) by only the quiescent cells with large and heterochromatinized nuclei that form the central core of 3D-sphere clusters. The cells were counterstained with PI to label the nuclei in red. All images are at ×400 magnification. Abbreviation: PI, propidium iodide.
When subconfluent cultures were pulsed with BrdU and analyzed after 30 minutes, we found BrdU-labeled cells clustering around but not within the spheres (supplemental online Fig. 5A). When we pulsed an early stage culture for 1 hour (at 4–5 days after suspension culture initiation, when small growing clones were observed) and chased for another 10 days, we observed label-retaining cells that were right within the 3D clusters and that were coexpressing C/EBPδ (supplemental online Fig. 5B). These observations suggest that the cells within the 3D clusters are quiescent stem cells. Quiescent stem cells are known to carry primary cilia to downregulate Wnt signaling and to establish reversible arrest. Consequently, we examined our cultures for the presence of ciliated cells using an antibody against acetylated tubulin. Interestingly, cells with large and heterochromatinized nuclei within 3D spheres distinctly expressed primary cilium, whereas it was completely missing from any of the surrounding epithelial cells (Fig. 6D). These observations suggest that the 3D compartments consist of ABCG2-, p63α-, and C/EBPδ-positive and low-Pax6-expressing quiescent stem cells surrounding a central core of ciliated cells with large, heterochromatinized nuclei and possibly other niche cells and ECM components.
Expression of Mesenchymal, Melanocyte, Neural Crest, and Neural Cell Markers
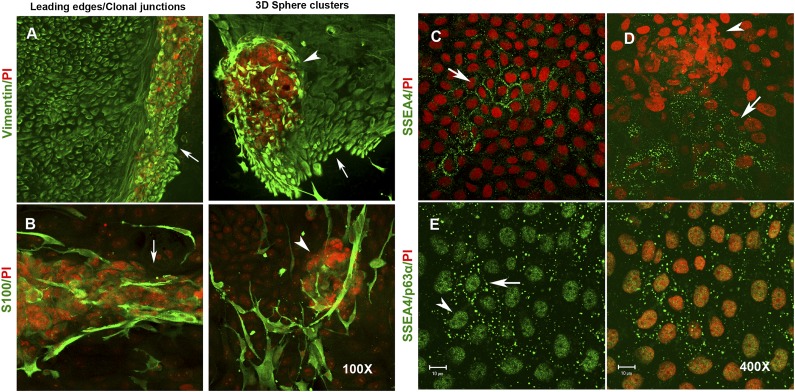
Apart from nestin, vimentin-positive mesenchymal-like niche cells were also found within and around the sphere clusters (Fig. 7A). The 3D spheres were often pigmented, and melanin-containing epithelial cells were visible around the spheres. This prompted us to check whether melanocytes were also expanded in our limbal cultures. Melanocytes are neural crest-derived cells and are known to express S100 [13, 33]. When suspension cultures were examined by ICC, we found S100-positive cells both within and surrounding the 3D spheres. Morphologically, S100-positive cells showed extended membrane projections that are typical of melanocytes and were found to be interspersed within the epithelial cell sheet (Fig. 7B). A few cells within the spheres showed weak cytosolic staining for tyrosinase, an enzyme involved in pigment biogenesis (data not shown). Because nestin is known to be expressed by neural progenitors, neural crest-derived cells, and some of the bone marrow-derived mesenchymal stem cells, we did coimmunostaining for both nestin and β-III tubulin (a neural progenitor-specific marker). We observed cells that coexpressed both proteins and those that exclusively expressed either of them (supplemental online Fig. 6). These observations clearly suggest the presence of neural progenitors in our limbal cultures, adding to the repertoire of niche cells found in vitro.
Figure 7.
Expression of stromal and melanocyte markers in 3D-sphere clusters. (A): Expression of vimentin (green) in growing epithelial cells and the stromal niche cells that migrate at the leading edge (left) and also within the 3D-sphere clusters (right). (B): Expression of S100 protein (green) within the pigmented 3D clusters at the colony junctions (left) and within the 3D-sphere clusters (right). Magnification, ×100 (A, B). Expression of SSEA4 in cultured limbal epithelial cells. (C): Expression of SSEA4 (green) in clusters of limbal epithelial cells. Note the clear membrane localization of the antigen. (D): Expression of SSEA4 in epithelial cells located immediately adjacent to a 3D-sphere cluster. (E): Coexpression of both SSEA4 (green, membrane) and p63α (green, nuclear) in limbal epithelial stem cells. Both the primary antibodies are mouse monoclonal and are labeled with anti-mouse Alexa Fluor 488. The cells are counter stained with PI (red). Magnification, ×400 (C–E). Abbreviations: 3D, three-dimensional; PI, propidium iodide.
Expression of Pluripotent Stem Cell Markers
Pluripotent or early progenitor cells expressing SSEA4, Oct4, Sox2, Nanog, and TRA antigens have been reported to be present in the limbal stroma. These SSEA4-positive limbal stromal cells are shown to be capable of differentiating into limbal epithelium and other cell lineages of the body [21, 29]. However, few reports show that SSEA4-positive limbal stromal cells do not express pluripotent stem cell markers [22, 23]. Another group reported that SSEA4-negative limbal cells are actually more clonogenic and are true stem cells [34]. Only two reports so far have shown clear nuclear localization of Oct4 in limbal stromal cells cultured under embryonic stem cell conditions [21, 29]. However, careful examination of other studies supports only cytosolic expression of Oct4, Sox2, and Nanog [35–37]. In our cultures, we found distinct patches of epithelial cells with clear membrane staining for SSEA4 (Fig. 7C) that were mostly located not within but in close proximity to the 3D spheres (Fig. 7D). These SSEA4-positive cell clusters also coexpressed p63α in the nucleus, suggesting that they are in fact epithelial progenitors but not stromal cells (Fig. 7E). We checked for the expression of other pluripotency markers Oct4 and Sox2. We observed that only sphere clusters showed clear cytosolic signals for anti-Oct4 and absolutely no signals elsewhere in the culture, possibly suggesting the presence of only low levels of inactive proteins (supplemental online Fig. 7). Sox2 expression showed a similar staining pattern (data not shown). Taken together, the results suggest that the limbal cultures have SSEA4-positive very early epithelial progenitors but not the pluripotent stem cells under our culture conditions.
Discussion
Earlier studies that comparatively evaluated limbal explant and suspension cultures had mainly examined the relative stem cell content and its expansion ability in vitro [9, 38]. In this paper, we report some of the similarities and the uniqueness of each of culture system in terms of the limbal stem cell and niche cell contents and their compartmentalization and localization in ex vivo cultures. Our results on FACS quantification of stem cells using markers such as Pax6, p63, and SSEA4 revealed no significant difference between the limbal explant and suspension cultures. This further explains the comparable CLET outcomes reported by two long-term clinical studies using these two culture methods [10, 11]. Both methods resulted in mixed limbal cultures consisting predominantly of epithelial cells along with some of the niche component cells. The niche cells includes the nestin- and vimentin-positive mesenchymal-like stromal cells, β-III tubulin-positive neural progenitors, and S100- and Tyr-positive neural crest-derived melanocytes. Our results also confirmed that CD29 is expressed by proliferating limbal epithelial cells but not stromal cells.
Interestingly, the niche cells are localized differently between the two culture systems. In case of nestin-positive cells, they are restricted to the explant edge in explant culture systems, whereas they migrate at the leading edges and are found within the 3D-sphere compartments in the suspension culture system. We believe that the niche cells within and adjacent to the explants maintain the putative stem cell pool and provide directional cues to guide the migration and proliferation of epithelial stem cells; however, this “position effect” is lost in early stage suspension cultures.
In near-confluent suspension cultures, both the epithelial stem cells and niche cells in culture seemed to interact with and regulate each other at clonal junctions. It is possible that the niche cells at the leading edges could be providing specific ECM and secretory molecules to signal and direct epithelial stem cell proliferation and migration. It also appeared that the different niche cells, together with stem cells and ECM components, carry an internal program to migrate in spiraling patterns and self-organize to form 3D compartments resembling the limbal crypts in vivo. The quiescent epithelial stem cells expressing high levels of p63α at the apical surface of the sphere clusters (section Z4, supplemental online Fig. 4B) could then be correlated to the deep limbal crypt stem cells. It is possible that such 3D compartments consisting of both the niche and stem cells could very well be an effort to restore the missing directional cues in mid- to late-stage suspension culture systems. Although it was exciting to watch the spiraling whorls of epithelial cells in culture dishes, it remains to be understood how these directional cues are communicated among the cells in two-dimensional culture environments.
In explant culture systems in which the directional cues are intact, the putative stem cells seem to reside close to the explant; however, the major pool of p15- and CEBPδ-positive and BrdU-label-retaining early activated but quiescent stem cells seems to migrate at the leading edges. In addition, stable cytosolic expression of p27 in migrating epithelial cells suggests the existence of an additional redundant mechanism for cell proliferation control at the leading edges. This observation confirms an earlier report [39] and raises an intriguing question as to why the activated stem cells should remain quiescent and migrate at the leading edge, away from the native stem cell niche (explant). We believe that the cells at the leading edge carry an inherent program (possibly ECM and cytokine signaling) to direct cell migration toward the site of injury. In addition, it is important that the leading edge cells should be able to gauge the extent of damage and dictate the proliferation of cells migrating behind. Once wound healing is complete and the leading edge cells from opposite ends meet and establish cell-cell contacts, the internal program should activate signaling cascades to inhibit any further cell migration and proliferation. It appears that the quiescent status of the leading edge cells serves as a foolproof mechanism devised to prevent the risk of uncontrolled cell proliferation during wound-healing response and ensure normal epithelial homeostasis.
Because the size of epithelial damage may be variable, it makes sense that subsets of initially activated stem cells remain quiescent and migrate at the leading edge and function as local reserves of the stem cell pool. In turn, they can become activated to give rise to more progenies based on local demand as the cells keep proliferating and migrating. If this were not the case, then the migratory cells at the leading edge would have to be in constant communication with the stem cells that are residing far away in the niche. In addition, the newly activated stem cells and their progenies have to migrate all the way from the niche to the leading edge to provide fresh supplies of cells. Consequently, the presence of quiescent stem cells at the leading edge appears to be an energy and stem cell resource conservation mechanism to enable complete regeneration of wounds, regardless of size. As reported previously [40, 41], it is possible that these early activated stem cells that migrate at the leading edges might contribute to the self-renewing potential of central corneal epithelium.
The expression of primary cilium by the cells with large and heterochromatinized nuclei within the 3D-sphere clusters could also serve as a novel marker to identify limbal stem cells. It is possible that the expression of primary cilia may have an important role in limbal stem cell signaling. It is well known that several cell-signaling molecules and receptors are localized to the ciliary membrane surface and help in sensing extracellular signals to regulate various cellular functions [42, 43]. When examined for the presence of pluripotent stem cells in limbal cultures, our observation confirmed that of earlier reports that SSEA4- and p63α-positive cells could be early limbal epithelial progenitors. Consequently, this marker combination could very well be used for the identification of putative limbal stem cells. However, we did not find proper nuclear expression of either Oct4 or Sox2. It remains to be studied whether the potency of limbal stem cells can be modulated under embryonic stem cell culture conditions.
Conclusion
Apart from limbal epithelial stem cells, we report that niche cells become expanded in limbal cultures. Although the niche cells appear to direct epithelial cell migration at leading edges in suspension cultures, they are limited to the native niche in explant cultures. We also provide evidence that the early activated stem cells remain quiescent and lead the epithelial cell migration in explants cultures. Furthermore, we report that the limbal stem and niche cells physically interact and self-organize to form 3D niche-like compartments in ex vivo suspension cultures. Finally, we conclude that both culture techniques could generate epithelial sheets containing the intact limbal niche, and it is important to retain the explants and 3D-sphere clusters while the engineered tissues are being used for CLET procedures.
Supplementary Material
Acknowledgments
This study was supported by grants to I.M. and V.S.S. from the Department of Biotechnology, Government of India; the Champalimaud Foundation, Portugal; and the Hyderabad Eye Research Foundation. J.P. was supported by a short-term Fulbright-Nehru student research grant. We thank Dr. D. Balasubramanian for critical review and useful suggestions in finalizing the manuscript.
Author Contributions
I.M.: conception and design, financial support, provision of study material, collection and/or assembly of data, data analysis and interpretation, manuscript writing, final approval of manuscript; S.K., J.P., S.M., and J.S.: collection and/or assembly of data, data analysis and interpretation; V.S.S.: provision of study material, financial support, final approval of manuscript.
Disclosure of Potential Conflicts of Interest
The authors indicate no potential conflicts of interest.
References
- 1.Schermer A, Galvin S, Sun TT. Differentiation-related expression of a major 64K corneal keratin in vivo and in culture suggests limbal location of corneal epithelial stem cells. J Cell Biol. 1986;103:49–62. doi: 10.1083/jcb.103.1.49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lavker RM, Sun TT. Epithelial stem cells: The eye provides a vision. Eye (Lond) 2003;17:937–942. doi: 10.1038/sj.eye.6700575. [DOI] [PubMed] [Google Scholar]
- 3.Dua HS, Shanmuganathan VA, Powell-Richards AO, et al. Limbal epithelial crypts: A novel anatomical structure and a putative limbal stem cell niche. Br J Ophthalmol. 2005;89:529–532. doi: 10.1136/bjo.2004.049742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Pellegrini G, Traverso CE, Franzi AT, et al. Long-term restoration of damaged corneal surfaces with autologous cultivated corneal epithelium. Lancet. 1997;349:990–993. doi: 10.1016/S0140-6736(96)11188-0. [DOI] [PubMed] [Google Scholar]
- 5.Tsai RJ, Li LM, Chen JK. Reconstruction of damaged corneas by transplantation of autologous limbal epithelial cells. N Engl J Med. 2000;343:86–93. doi: 10.1056/NEJM200007133430202. [DOI] [PubMed] [Google Scholar]
- 6.Koizumi NJ, Inatomi TJ, Sotozono CJ, et al. Growth factor mRNA and protein in preserved human amniotic membrane. Curr Eye Res. 2000;20:173–177. [PubMed] [Google Scholar]
- 7.Sangwan VS, Matalia HP, Vemuganti GK, et al. Clinical outcome of autologous cultivated limbal epithelium transplantation. Indian J Ophthalmol. 2006;54:29–34. doi: 10.4103/0301-4738.21611. [DOI] [PubMed] [Google Scholar]
- 8.Mariappan I, Maddileti S, Savy S, et al. In vitro culture and expansion of human limbal epithelial cells. Nat Protoc. 2010;5:1470–1479. doi: 10.1038/nprot.2010.115. [DOI] [PubMed] [Google Scholar]
- 9.Koizumi N, Cooper LJ, Fullwood NJ, et al. An evaluation of cultivated corneal limbal epithelial cells, using cell-suspension culture. Invest Ophthalmol Vis Sci. 2002;43:2114–2121. [PubMed] [Google Scholar]
- 10.Rama P, Matuska S, Paganoni G, et al. Limbal stem-cell therapy and long-term corneal regeneration. N Engl J Med. 2010;363:147–155. doi: 10.1056/NEJMoa0905955. [DOI] [PubMed] [Google Scholar]
- 11.Sangwan VS, Basu S, Vemuganti GK, et al. Clinical outcomes of xeno-free autologous cultivated limbal epithelial transplantation: A 10-year study. Br J Ophthalmol. 2011;95:1525–1529. doi: 10.1136/bjophthalmol-2011-300352. [DOI] [PubMed] [Google Scholar]
- 12.Gage PJ, Rhoades W, Prucka SK, et al. Fate maps of neural crest and mesoderm in the mammalian eye. Invest Ophthalmol Vis Sci. 2005;46:4200–4208. doi: 10.1167/iovs.05-0691. [DOI] [PubMed] [Google Scholar]
- 13.Yoshida S, Shimmura S, Nagoshi N, et al. Isolation of multipotent neural crest-derived stem cells from the adult mouse cornea. Stem Cells. 2006;24:2714–2722. doi: 10.1634/stemcells.2006-0156. [DOI] [PubMed] [Google Scholar]
- 14.Niederer RL, Perumal D, Sherwin T, et al. Corneal innervation and cellular changes after corneal transplantation: An in vivo confocal microscopy study. Invest Ophthalmol Vis Sci. 2007;48:621–626. doi: 10.1167/iovs.06-0538. [DOI] [PubMed] [Google Scholar]
- 15.Hayashi R, Yamato M, Sugiyama H, et al. N-cadherin is expressed by putative stem/progenitor cells and melanocytes in the human limbal epithelial stem cell niche. Stem Cells. 2007;25:289–296. doi: 10.1634/stemcells.2006-0167. [DOI] [PubMed] [Google Scholar]
- 16.Li W, Hayashida Y, Chen YT, et al. Niche regulation of corneal epithelial stem cells at the limbus. Cell Res. 2007;17:26–36. doi: 10.1038/sj.cr.7310137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Secker GA, Daniels JT. Limbal epithelial stem cells of the cornea. Available at http://www.stembook.org/node/588 Accessed May 9, 2014. [PubMed]
- 18.Li GG, Zhu YT, Xie HT, et al. Mesenchymal stem cells derived from human limbal niche cells. Invest Ophthalmol Vis Sci. 2012;53:5686–5697. doi: 10.1167/iovs.12-10300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Li GG, Chen SY, Xie HT, et al. Angiogenesis potential of human limbal stromal niche cells. Invest Ophthalmol Vis Sci. 2012;53:3357–3367. doi: 10.1167/iovs.11-9414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Du Y, Funderburgh ML, Mann MM, et al. Multipotent stem cells in human corneal stroma. Stem Cells. 2005;23:1266–1275. doi: 10.1634/stemcells.2004-0256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Dravida S, Pal R, Khanna A, et al. The transdifferentiation potential of limbal fibroblast-like cells. Brain Res Dev Brain Res. 2005;160:239–251. doi: 10.1016/j.devbrainres.2005.09.008. [DOI] [PubMed] [Google Scholar]
- 22.Polisetty N, Fatima A, Madhira SL, et al. Mesenchymal cells from limbal stroma of human eye. Mol Vis. 2008;14:431–442. [PMC free article] [PubMed] [Google Scholar]
- 23.Lim MN, Hussin NH, Othman A, et al. Ex vivo expanded SSEA-4+ human limbal stromal cells are multipotent and do not express other embryonic stem cell markers. Mol Vis. 2012;18:1289–1300. [PMC free article] [PubMed] [Google Scholar]
- 24.Seigel GM, Sun W, Salvi R, et al. Human corneal stem cells display functional neuronal properties. Mol Vis. 2003;9:159–163. [PubMed] [Google Scholar]
- 25.Uchida S, Yokoo S, Yanagi Y, et al. Sphere formation and expression of neural proteins by human corneal stromal cells in vitro. Invest Ophthalmol Vis Sci. 2005;46:1620–1625. doi: 10.1167/iovs.04-0288. [DOI] [PubMed] [Google Scholar]
- 26.Hashmani K, Branch MJ, Sidney LE, et al. Characterization of corneal stromal stem cells with the potential for epithelial transdifferentiation. Stem Cell Res Ther. 2013;4:75. doi: 10.1186/scrt226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Yoshida S, Shimmura S, Shimazaki J, et al. Serum-free spheroid culture of mouse corneal keratocytes. Invest Ophthalmol Vis Sci. 2005;46:1653–1658. doi: 10.1167/iovs.04-1405. [DOI] [PubMed] [Google Scholar]
- 28.Mimura T, Yamagami S, Uchida S, et al. Isolation of adult progenitor cells with neuronal potential from rabbit corneal epithelial cells in serum- and feeder layer-free culture conditions. Mol Vis. 2010;16:1712–1719. [PMC free article] [PubMed] [Google Scholar]
- 29.Katikireddy KR, Dana R, Jurkunas UV. Differentiation potential of limbal fibroblasts and bone marrow mesenchymal stem cells to corneal epithelial cells. Stem Cells. 2014;32:717–729. doi: 10.1002/stem.1541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Collinson JM, Morris L, Reid AI, et al. Clonal analysis of patterns of growth, stem cell activity, and cell movement during the development and maintenance of the murine corneal epithelium. Dev Dyn. 2002;224:432–440. doi: 10.1002/dvdy.10124. [DOI] [PubMed] [Google Scholar]
- 31.Nagasaki T, Zhao J. Centripetal movement of corneal epithelial cells in the normal adult mouse. Invest Ophthalmol Vis Sci. 2003;44:558–566. doi: 10.1167/iovs.02-0705. [DOI] [PubMed] [Google Scholar]
- 32.Mort RL, Ramaesh T, Kleinjan DA, et al. Mosaic analysis of stem cell function and wound healing in the mouse corneal epithelium. BMC Dev Biol. 2009;9:4. doi: 10.1186/1471-213X-9-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Nubile M, Lanzini M, Calienno R, et al. S100 A and B expression in normal and inflamed human limbus. Mol Vis. 2013;19:146–152. [PMC free article] [PubMed] [Google Scholar]
- 34.Truong TT, Huynh K, Nakatsu MN, et al. SSEA4 is a potential negative marker for the enrichment of human corneal epithelial stem/progenitor cells. Invest Ophthalmol Vis Sci. 2011;52:6315–6320. doi: 10.1167/iovs.11-7518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Zhou SY, Zhang C, Baradaran E, et al. Human corneal basal epithelial cells express an embryonic stem cell marker OCT4. Curr Eye Res. 2010;35:978–985. doi: 10.3109/02713683.2010.516465. [DOI] [PubMed] [Google Scholar]
- 36.Chen SY, Hayashida Y, Chen MY, et al. A new isolation method of human limbal progenitor cells by maintaining close association with their niche cells. Tissue Eng Part C Methods. 2011;17:537–548. doi: 10.1089/ten.tec.2010.0609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Xie HT, Chen SY, Li GG, et al. Limbal epithelial stem/progenitor cells attract stromal niche cells by SDF-1/CXCR4 signaling to prevent differentiation. Stem Cells. 2011;29:1874–1885. doi: 10.1002/stem.743. [DOI] [PubMed] [Google Scholar]
- 38.Zhang X, Sun H, Tang X, et al. Comparison of cell-suspension and explant culture of rabbit limbal epithelial cells. Exp Eye Res. 2005;80:227–233. doi: 10.1016/j.exer.2004.09.005. [DOI] [PubMed] [Google Scholar]
- 39.Zieske JD, Francesconi CM, Guo X. Cell cycle regulators at the ocular surface. Exp Eye Res. 2004;78:447–456. doi: 10.1016/s0014-4835(03)00205-7. [DOI] [PubMed] [Google Scholar]
- 40.Majo F, Rochat A, Nicolas M, et al. Oligopotent stem cells are distributed throughout the mammalian ocular surface. Nature. 2008;456:250–254. doi: 10.1038/nature07406. [DOI] [PubMed] [Google Scholar]
- 41.Dua HS, Miri A, Alomar T, et al. The role of limbal stem cells in corneal epithelial maintenance: Testing the dogma. Ophthalmology. 2009;116:856–863. doi: 10.1016/j.ophtha.2008.12.017. [DOI] [PubMed] [Google Scholar]
- 42.Davis EE, Brueckner M, Katsanis N. The emerging complexity of the vertebrate cilium: New functional roles for an ancient organelle. Dev Cell. 2006;11:9–19. doi: 10.1016/j.devcel.2006.06.009. [DOI] [PubMed] [Google Scholar]
- 43.Bisgrove BW, Yost HJ. The roles of cilia in developmental disorders and disease. Development. 2006;133:4131–4143. doi: 10.1242/dev.02595. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.