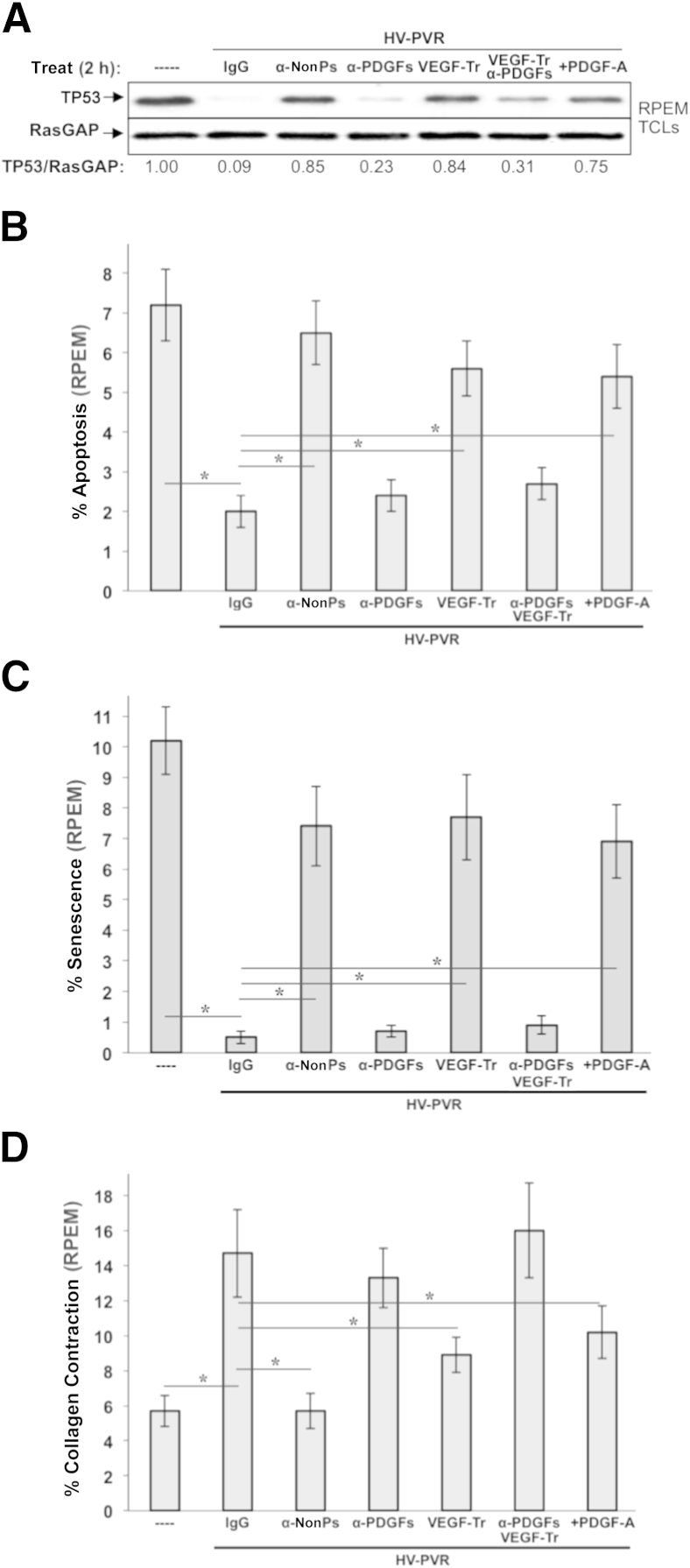
Figure 8.
Vascular endothelial growth factor (VEGF) and non–platelet-derived growth factors (non-PDGFs) enhance, whereas PDGFs antagonize, the bioactivity of vitreous from patients with proliferative vitreoretinopathy (PVR) after severe trauma. A: Non-PDGFs and VEGF are necessary for vitreous from patients with PVR after severe trauma (HV-PVR) to reduce TP53 in retinal pigment epithelial (RPE) cells isolated from a human PVR membrane (RPEM cells). RPEMs were starved and then either left untreated (----) or treated for 2 hours as indicated in the presence of HV-PVR—an equal volume mix of 10 vitreous samples from patients with post-traumatic grade C PVR. Treatments included 10 μg/mL of nonimmune IgG (IgG), a mixture of neutralizing antibodies directed against the 13 non-PDGFs included in GFs-PVR as listed in Table 2 (α-nonPs), an equimolar mixture of neutralizing antibodies against all PDGF agonists of PDGFRα (α-PDGFs), 250 μg/μL of aflibercept/VEGF-Trap (VEGF-Tr), a combination of VEGF-Tr + α-PDGFs (same concentrations used individually), and a supersaturating dose of 500 ng/mL PDGF-A (+PDGF-A). After treatment, cells were lysed and the resulting total cell lysates were subjected to Western blot analysis using the indicated antibodies. The signal intensity of the resulting immunoblots was quantified by densitometry, and ratios representing band intensities normalized to non-stimulated cells are shown under the immunoblot. B: Non-PDGFs and VEGF were necessary for HV-PVR's ability to protect RPEMs from starvation-induced apoptosis. RPEMs were starved 24 hours and then administered the same treatments as in A except treatment was maintained for 72 hours instead of 2 hours, with starvation media plus supplements replaced daily. At 96 hours, cells were subjected to TUNEL analysis. The data shown represent the means ± SD of three independent experiments. Data were compared using a paired t-test. Endogenous PDGF contributes a small amount to HV-PVR–driven contraction; elevating the concentration of PDGFs in HV-PVR can also drive contraction. Both these results are expected because PDGF itself can drive contraction,53,54 which is much more apparent at earlier time points (<4 days, data not shown). C: Non-PDGFs and VEGF are necessary for HV-PVR’s ability to protect RPEMs from starvation-induced senescence. RPEMs were starved 24 hours and then administered the same treatments as in A except treatment was maintained for 72 hours instead of 2 hours, with starvation media plus supplements replaced daily. The percentage of senescent cells was determined at 96 hours. The data shown represent the means ± SD from three independent experiments, and were statistically compared as in B. D: Non-PDGFs and VEGF were necessary for RPEM-mediated collagen gel contraction driven by HV-PVR. RPEMs were starved, preconditioned for 24 hours with the same treatments in A, and then suspended in a collagen gel and treated an additional 72 hours, with starvation media plus supplements replaced daily. Gel area was measured at 96 hours to determine the percentage collagen contraction. Each bar represents a mean ± SD percentage contraction obtained from three independent experiments, and was statistically compared as in B. ∗P < 0.05.