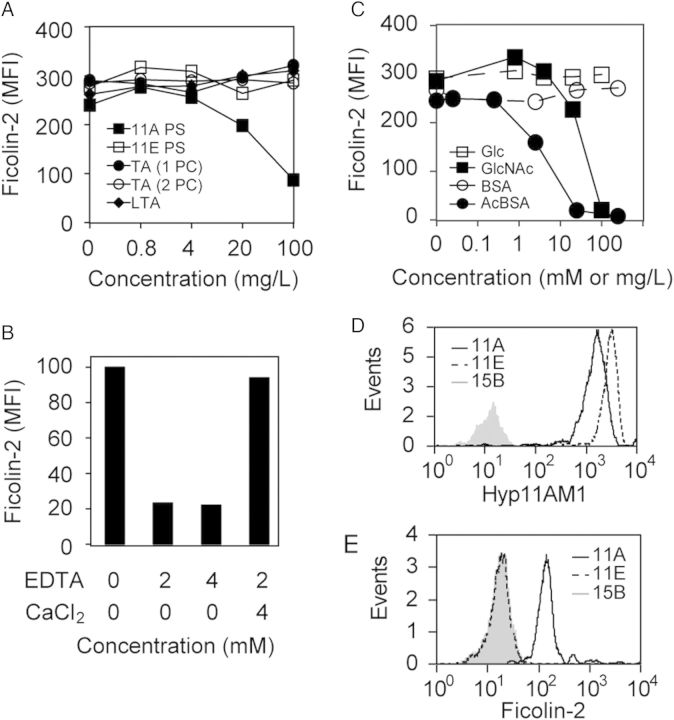
Figure 2.
Specificity of ficolin-2 binding to ST11A bacteria was examined by flow cytometry and expressed as mean fluorescence intensity (MFI). All graphs are representative of at least 3 experiments. A, Serum ficolin-2 binding to ST11A pneumococci in the presence of serially diluted purified polysaccharide (PS) preparations: ST11A PS (solid squares), ST11E PS (open squares), teichoic acid with 1 phosphocholine per repeat unit (TA 1PC; solid circles), teichoic acid with 2 PCs per repeat unit (TA 2PC; open circles), or lipoteichoic acid (LTA; solid diamonds). B, Serum ficolin-2 binding to JC03 incubated in 2.5% serum in the presence of specified concentrations (mM) of ethylenediaminetetraacetic acid (EDTA) and CaCl2. C, Serum ficolin-2 binding to ST11A pneumococci in the presence of inhibitors: glucose (Glc; open squares), bovine serum albumin (BSA; open circles), N-acetylglucosamine (GlcNAc; solid squares), or acetylated-BSA (acBSA; solid circles). Inhibitor concentrations are mM for Glc and GlcNAc and mg/L for BSA and acBSA. D–E, Flow cytometric detection of ficolin-2 binding to ST11A polysaccharide (PS)–coated latex beads. Data are representative of at least 3 experiments. D, Latex beads were conjugated with purified ST11A PS (solid black curves), ST11E PS (dotted black curves), or 15B PS (gray curves) and were examined for ST11A/11E PS, using Hyp11AM1 monoclonal antibody. E, The same PS-conjugated latex beads used in panel D were incubated in 0.17% normal human serum and examined for ficolin-2 binding, using anti-ficolin-2 mAb GN5.