Abstract
Background
Within a large prospective study, the Global Asthma and Allergy European Network (GA2LEN) has collected skin prick test (SPT) data throughout Europe to make recommendations for SPT in clinical settings.
Objective
To improve clinical interpretation of SPT results for inhalant allergens by providing quantitative decision points.
Methods
The GA2LEN SPT study with 3068 valid data sets was used to investigate the relationship between SPT results and patient-reported clinical relevance for each of the 18 inhalant allergens as well as SPT wheal size and physician-diagnosed allergy (rhinitis, asthma, atopic dermatitis, food allergy). The effects of age, gender, and geographical area on SPT results were assessed. For each allergen, the wheal size in mm with an 80% positive predictive value (PPV) for being clinically relevant was calculated.
Results
Depending on the allergen, from 40% (blatella) to 87–89% (grass, mites) of the positive SPT reactions (wheal size ≥ 3 mm) were associated with patient-reported clinical symptoms when exposed to the respective allergen. The risk of allergic symptoms increased significantly with larger wheal sizes for 17 of the 18 allergens tested. Children with positive SPT reactions had a smaller risk of sensitizations being clinically relevant compared with adults. The 80% PPV varied from 3 to 10 mm depending on the allergen.
Conclusion
These ‘reading keys’ for 18 inhalant allergens can help interpret SPT results with respect to their clinical significance. A SPT form with the standard allergens including mm decision points for each allergen is offered for clinical use.
Keywords: allergy diagnostics, common allergens, sensitization, skin prick test
Introduction
Skin prick testing (SPT) is the standard method world-wide to assess IgE-mediated sensitization to allergens 1. It is often the first method used for screening of possible causative agents in subjects with symptoms suggesting allergy. It is a rapid, reproducible, and accurate way of identifying allergens in IgE-mediated allergy, but the interpretation of the test results in relation to the subject′s symptoms requires experience. While a negative skin prick test response has a good negative predictive value for excluding the presence of an IgE-mediated reaction, an isolated positive response is not a proof of a clinically relevant allergy 2.
The fact that sensitizations are not always accompanied by clinical symptoms is well known 3 and part of the self-regulation of the immune system. However, inexperienced clinicians and patients tend to interpret positive SPT results as diagnosed allergy requiring actions, including, for example, guidance for avoidance, medication, and even immunotherapy. Such actions may not be adequate. Incorrect interpretation of positive SPT can result in overdiagnosis of allergic disorders and medicalization. A particularly poor relationship between positive SPT results and clinical symptoms appears to exist for food allergens 4,5. For inhalant allergens, the clinical relevance has been insufficiently investigated.
The Global Allergy and Asthma European Network (GA2LEN) was created in 2005 to ensure excellence in research bringing together research and clinical institutions to combat fragmentation in the European research area and to tackle allergy 6. A survey a few years ago showed that the procedures for skin prick testing in Europe varied a lot 7. A subsequent European-wide GA2LEN SPT study assessed more than 3000 patients with a standardized prick test procedure and standardized inhalant allergens 8 and resulted in a recommendation for standardized skin prick testing 9. Further analysis of this data showed that eight to ten common allergens allowed the identification of the majority of sensitized subjects, as a result of cross-reacting allergens to primary sensitizing allergens 10. This is useful for epidemiological studies. For clinical care of individual patients, however, the panel of 18 allergens is needed to appropriately assess sensitization across Europe. This is based on the consensus previously made for contact allergy to include those allergens in a standard series showing sensitization in at least 2% of the subjects. Testing of additional allergens may be required based on individual exposure, especially with animal dander and in occupational settings.
As the interpretation of SPT results is crucial for correct treatment decisions, this study aimed at developing ‘reading keys’ to assist in the interpretation of test results.
Methods
Standardized skin prick testing was performed at 17 centres in 14 European countries as reported before 8. A total of 3068 patients were included: 84% adults, 60% female, mean age 34.5 years; 16% children, 40% female, mean age 10.3 years. They were consulting an outpatient clinic at one of the study centres because of a suspected IgE-mediated allergic condition. The allergen extracts used were as follows: grass mix, Alternaria alternata, Parietaria, cat, dog, Ambrosia, positive control, negative control (ALK-Scherax, Hamburg, Germany), Cladosporium herbarum, Aspergillus fumigatus, birch, hazel, alder (Allergopharma, Reinbek, Germany), Blatella/Blatella germanica (Leti Pharma, Witten, Germany), Dermatophagoides pteronyssinus (Dp), Dermatophagoides farinae, plane, Artemisia, olive, cypress/Cupressus sempervirens (Stallergenes, Kamp-Lintfort, Germany). The largest and perpendicular diameter of the wheal elicited by the allergens was measured and mean value (D+d/2) calculated. Wheal reactions with a mean diameter of 3 mm or more were regarded as positive, if the control solutions showed expected result (wheal size at least 3 mm for the positive control, histaminedihydrochloride 10 mg/mL, and less than 3 mm for the negative control, allergen solvent). Patient was excluded if the responses to control solutions were not adequate.
Among the subjects tested, a total of 2088 (68%) showed at least one 3-mm wheal reaction to the 18 allergens employed, and in 980 patients (32%), all wheals were less than 3 mm. When the SPT result was read, the clinicians (allergists) asked for the clinical relevance of the allergen causing a wheal reaction of 3 mm or more (0 = not relevant, no related allergic symptoms; 1 = relevant, related allergic symptoms; 2 = former relevance, previously related allergic symptoms; 3 = unknown). For the analysis, the clinical relevance was defined as current relevance or former relevance. Nasal, conjunctival, lower airway, skin, and gastrointestinal symptoms were considered. The severity of the symptoms was not asked for. The original standard operating procedure (SOP) explaining this to the participating investigators is attached as a supplemental file. The relationship between the SPT wheal size and the clinical relevance of the allergen in question was investigated.
There were 1888 subjects who showed a positive SPT to at least one of six allergens with the highest prevalence of positive reactions (hazel, birch, grass, cat, dog, and Dp; the reactions to the two mites were concordant in 80%). These subjects were grouped based on physicians' diagnoses: allergic rhinitis (79%), allergic asthma (36%), atopic dermatitis (19%), and food allergy (13%). Obviously, many patients reported several diagnoses: 29% reported two, 8% three, and 3% all four diagnoses. The relationship between wheal sizes and the four clinical diagnoses mentioned above was established.
To show regional specificities, three groups of patients were formed based on climatic data 8: a Nordic group (Denmark, Finland), a Mediterranean group (Greece, France, Italy, Portugal) and a Central European group (Austria, Belgium, Germany, Hungary, the Netherlands, Poland, Switzerland, United Kingdom).
In the age-specific analysis, patients were divided into children less than 15 years and adults. Patients were also analysed based on gender.
Statistical analysis
To illustrate the relationship between the wheal size and the symptoms in SPT-positive patients, a logistic regression analysis was adapted with the dichotomous symptom variable as an outcome and the wheal size as a predictor. A graph with the predicted probabilities and their 95% confidence intervals was created, and the odds ratios (with 95% CI) for the wheal size to the allergen in question were reported. Chi-square test was used to test frequencies between groups of SPT-positive patients.
To investigate the relationships between the diagnosis and the six most frequently positive allergens simultaneously, we included all patients into a multivariate logistic regression model with gender, childhood, and six dichotomous test results for the allergens as predictors. The α-level (statistical significance) was set at 0.05 for all tests.
Results
The positive control, histaminedihydrochloride 10 mg/mL, gave on average 6.1 (SD 2.0) mm wheal response, 5.9 mm in children (SD 2.1), and 6.2 mm in adults (SD 2.0). The total number of positive SPT results to allergens was 9171. Of these, 5564 (61%) were clinically relevant, 290 (3%) indicated former relevance, 1822 (20%) were not clinically relevant, and relevance was unknown for 1495 (16%).
The relationship between SPT wheal sizes and the percentage of patients with allergic symptoms are shown for all 18 allergens (Fig.1). In general, with increasing wheal sizes, the prevalence of allergic symptoms increased. Depending on the allergen, from 40% (blatella) to 87–89% (grass, Dp) of the positive SPT reactions (3 mm or more) were associated with patient-reported clinical symptoms. The relationship between the allergic symptoms and the wheal size in each of these 18 allergens expressed as crude odds ratios is shown in Table1. With the exception of Aspergillus fumigatus, a larger wheal size significantly increased the risk of having symptoms.
Figure 1.
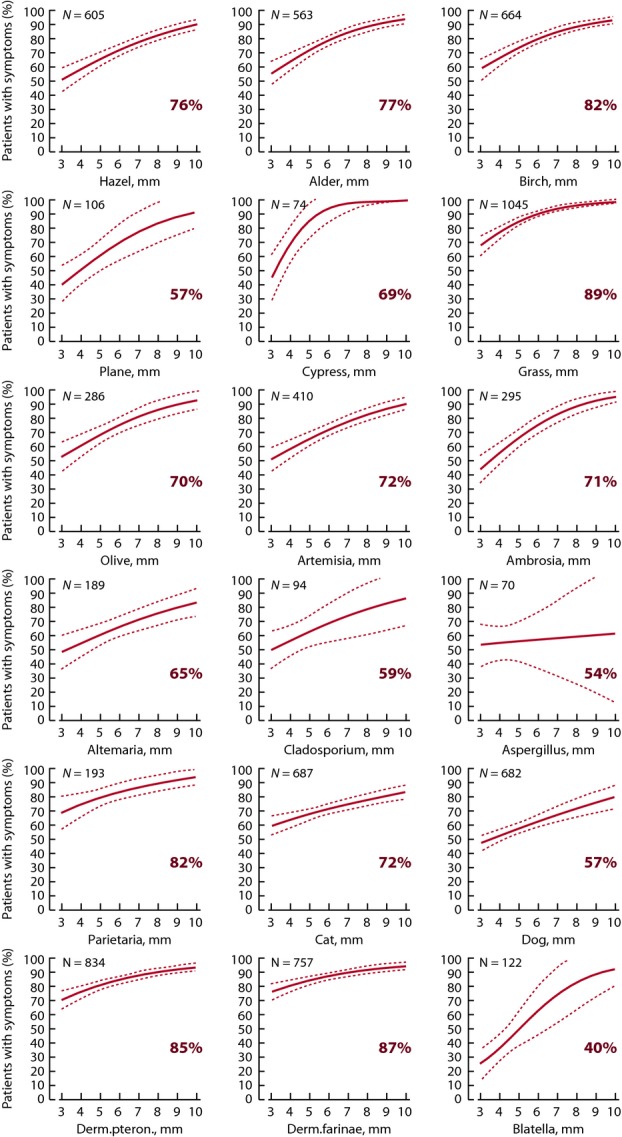
Clinical relevance of positive SPT reactions (wheal size 3 mm or more) of 18 common inhalant allergens in Europe. Solid line shows the estimated proportion of patients with symptoms and dotted lines 95% confidence intervals. Positive predictive value (%) is shown in each panel.
Table 1.
The panel of eighteen allergens used in skin prick testing (SPT). Univariate logistic regression analysis for clinical relevance in SPT-positive patients (wheal size 3 mm or larger) and 80% positive predictive value (PPV) for SPT wheal diameter for each of the 18 allergens. PPV for Aspergillus was not calculated because of the small number of positive results
| Allergen | Manufacturer and label of allergen potency (concentration) | Number of observations included in analyses | Odds Ratio* (95% CI) | P-value | 80% PPV for SPT wheal diameter, mm |
|---|---|---|---|---|---|
| Hazel | AL 50 000 BU/mL | 605 | 1.36 (1.25–1.48) | <0.001 | 5 |
| Alder | AL 50 000 BU/mL | 563 | 1.43 (1.29–1.60) | <0.001 | 4 |
| Birch | AL 50 000 BU/mL | 664 | 1.45 (1.32–1.60) | <0.001 | 3 |
| Plane | ST 100 IC/mL | 101 | 1.50 (1.13–2.00) | 0.005 | 7 |
| Cypress | ST 100 IC/mL | 74 | 2.70 (1.38–5.27) | 0.004 | 4 |
| Grass mix | AA 10 HEP | 1045 | 1.62 (1.45–1.82) | <0.001 | 3 |
| Olive | ST 100 IR/mL | 286 | 1.40 (1.21–1.63) | <0.001 | 6 |
| Artemisia | AA 1:100 W/V | 410 | 1.37 (1.24–1.51) | <0.001 | 5 |
| Ambrosia | AA 1:100 W/V | 295 | 1.58 (1.36–1.82) | <0.001 | 5 |
| Alternaria | AA 1:20 W/V | 189 | 1.27 (1.10–1.45) | 0.001 | 8 |
| Cladosporium | AL 10 000 BU/mL | 94 | 1.30 (1.00–1.68) | 0.049 | 7 |
| Aspergillus | AL 10 000 BU/mL | 70 | 1.05 (0.75–1.46) | 0.78 | ND |
| Parietaria | AA 10 HEP | 193 | 1.31 (1.11–1.56) | 0.002 | 3 |
| Cat | AA 10 HEP | 687 | 1.19 (1.10–1.29) | <0.001 | 7 |
| Dog | AA 10 HEP | 682 | 1.24 (1.13–1.35) | <0.001 | 10 |
| Derm. pteron. | ST 100 IR/mL | 834 | 1.32 (1.20–1.45) | <0.001 | 3 |
| Derm.farinae | ST 100 IR/mL | 757 | 1.29 (1.15–1.44) | <0.001 | 3 |
| Blatella | LE 1 mg/mL | 122 | 1.69 (1.19–2.38) | 0.003 | 7 |
OR for wheal size (mm), ND not done.
AA, ALK-Abello, AL, Allergopharma, LE, Leti, ST, Stallergenes. HEP, histamine equivalent prick; W/V, weight/volume; BU, biological units; IR, index of reactivity; IC, index of concentration.
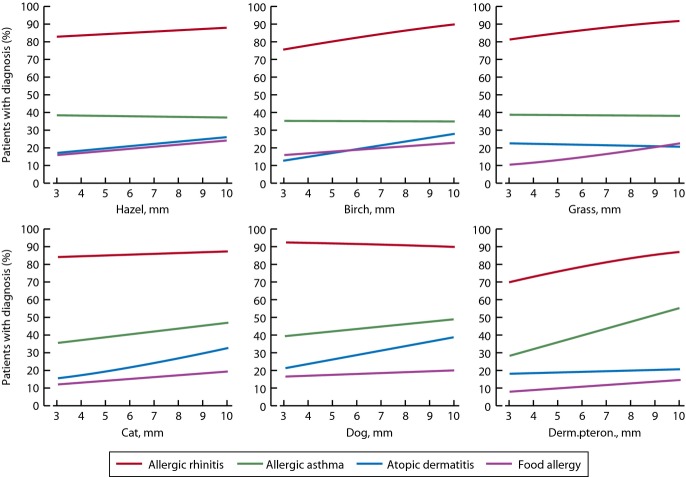
The wheal sizes of SPT with hazel, birch, grass, cat, dog, and Dp in relation to the percentage of patients with physician-diagnosed allergic rhinitis, allergic asthma, atopic dermatitis, and food allergy are shown in Fig.2. Overall, in patients with positive SPTs, and one or more diagnosis, allergic rhinitis was by far the most prevalent disorder – 80–90% of SPT-positive patients had rhinitis. Allergic asthma was present in 30–40% and atopic dermatitis or food allergies in 10–20% of those with positive SPTs. For Dp, a clear increase in diagnosed asthma and rhinitis was seen with larger wheal sizes (Fig.2). Similarly, larger wheal sizes in SPT for birch, cat, and dog were associated with higher rates of atopic dermatitis (Fig.2).
Figure 2.
Clinical relevance of SPT reactions according to physician-made diagnosis: allergic rhinitis, allergic asthma, atopic dermatitis, and food allergy. Solid line shows the estimated proportion of patients with diagnosis. X-axis shows the diameter of the SPT wheal in mm.
The relationships between each diagnosis (rhinitis, allergic asthma, atopic dermatitis, and food allergy) and sensitization to these six allergens shown in Fig.2 were investigated simultaneously using logistic regression with gender and childhood as covariates. A positive SPT reaction to grass (OR 2.96, 95% CI 2.4–3.7), cat (OR 2.0, CI 1.6–2.6), Dp (OR 1.7, CI 1.4–2.1) and hazel (OR 1.7, CI 1.1–2.5) significantly increased the risk of rhinitis. A positive SPT reaction to Dp (OR 2.2, 95% CI 1.8–2.6), cat (OR 1.4, CI 1.1–1.8), and grass (OR 1.2, CI 1.0–1.5) increased significantly the risk of allergic asthma, but especially being a child (OR 4.2, CI 3.4–5.2). A positive SPT reaction to cat (OR 1.3, 95% CI 1.0–1.7) and grass (OR 1.3, CI 1.0–1.6) increased slightly the dermatitis risk, but especially being a child (OR 1.5, CI 1.2–1.9) or female (OR 1.5, CI 1.2–1.9). For food allergy, a positive SPT reaction of birch (OR 1.7, CI 1.1–2.6) and female gender (OR 1.4, CI 1.1–1.8) increased the risk.
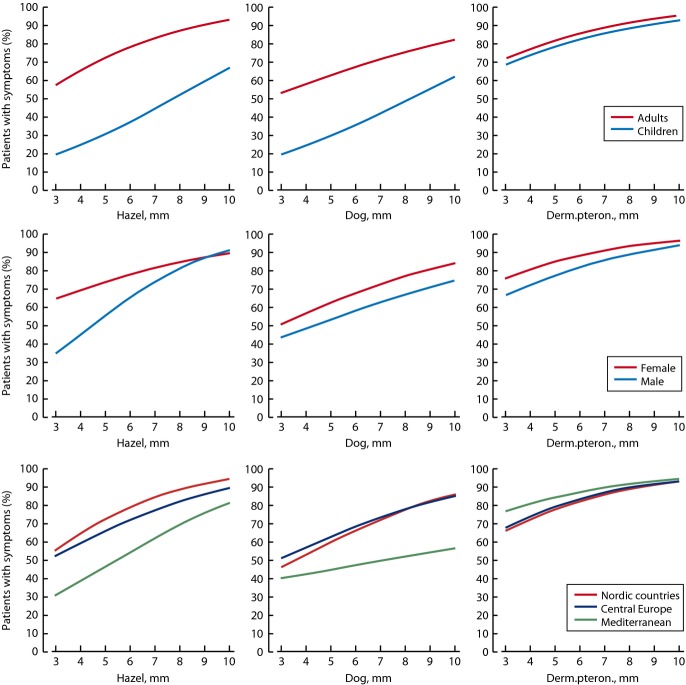
To determine differences in clinical relevance of sensitizations subgroup, analyses were performed by age, gender, and region. The prevalence of allergic symptoms for the subgroups is shown for hazel, dog, and Dp (Fig.3), the three allergens with enough positive reactions to make subgroup analysis. SPT-positive children were clearly less frequently symptomatic than SPT-positive adults in relation to hazel (42 vs. 81%, P < 0.001) and dog allergens (28 vs. 61%, P < 0.001), with no difference in relation to house dust mites (Fig.3). However, the relationship of more frequent symptoms in case of larger wheals remained. The frequency of symptoms was slightly higher among SPT-positive women compared with SPT-positive men in relation to wheal sizes for hazel (80 vs. 71%, P = 0.012), dog (61 vs. 52%, P = 0.031), and Dp (87 vs. 83%, P = 0.064) (Fig.3). In the regional analyses, there was a lower frequency of symptoms in the Mediterranean vs. Nordic or Central European regions in relation to positive SPTs to hazel and dog allergens (Fig.3). No regional differences were found for grass or house dust mite.
Figure 3.
Clinical relevance of SPT reactions for hazel, dog, and Dermatophagoides pteronyssinus by age group, gender, and region. Solid line shows the estimated proportion of patients with clinical symptoms. X-axis shows the diameter of the SPT wheal in mm.
Discussion
Based on the data of the European GA2LEN skin prick test study, a strong correlation between skin prick test (SPT) wheal sizes in 17 of the 18 allergens tested and the patient-reported allergy symptoms on exposure to the allergen in question was shown. Secondly, positive SPTs correlated particularly well with physician-diagnosed allergic rhinitis, and the correlation was poorer for asthma and poorest for atopic dermatitis and food allergy. This is an expected finding as rhinoconjunctivitis is most often triggered by IgE-mediated sensitization while in asthma other mechanisms, such as predisposition to increased bronchial responsiveness, play a major role. Sensitization to inhalant allergens seems to play only a minor role in patients with atopic dermatitis and food allergy, except those sensitized to major birch pollen allergen, Bet v 1, and/or profilin. It should be noted, however, that ingested allergens were not studied. Furthermore, the population tested came from specialist departments, reflecting expert practices in Europe.
The differences between children and adults may reflect differences in exposure although immunological factors are also involved as histamine reactions are also slightly smaller in children. However, as exposure to allergens is a function of time, it is predictable that young individuals, not exposed to allergens to the same extent as older, have a lower prevalence of positive SPTs and smaller wheal sizes. Studies also show that the frequency of asthma and allergic rhinitis continues to increase as the subjects become older 11.
A small gender difference was observed, with women reporting more often symptoms than men in relation to the SPT results. This might reflect more ready symptom reporting rather than more prevalent IgE-mediated conditions 12.
When SPT results were grouped according to geographical regions – the Nordic, Central European, and Southern European countries – differences were found indicating variable exposure to sensitizing inhalant allergens. The biggest difference was seen for broad-leaf trees (birch, alder, hazel) with a scarce occurrence in the Mediterranean countries. No differences of frequency of symptom were seen for house dust mites between regions.
Skin prick testing should be a standardized procedure with standardized allergens 7,9. European recommendations have been available for more than two decades 13, but high variations in testing practices, interpretations, and use of allergens have been observed 3,7,14. For example, SPT results obtained with the same allergen with extracts from different manufacturers vary 15–20. In the study reported here, the same commercially available extracts were used across all centres, and SPTs were performed according to the same standard operating procedure. This standardization and cautious clinical interpretation of test results are important as positive SPTs, particularly with small wheal sizes, have a poor positive predictive value (or strong negative predictive value if negative) for clinical symptoms caused by the allergen in question 13,21.
It should be noted that clinical relevance was not asked, if wheal sizes were less than 3 mm, and serum IgE measurements were not made even though smaller wheals (< 3 mm) have occasionally correlated to allergen-specific IgE values 22. Most individuals who get into contact with proteins capable of inducing an IgE response get sensitized and produce low amounts of allergen-specific IgE antibodies, which are detectable in serum 23. Atopic subjects are characterized by developing higher levels of IgE antibodies when exposed to low doses of allergens 13. These subjects when skin prick tested react more often with larger wheal sizes. However, only some of them show clinical symptoms when exposed to the allergen in question. The cross-reaction patterns of various allergens should also be noted. The SPT panel contained allergens biologically closely related such as Artemisia/Ambrosia, hazel/alder/birch, D. pteronyssinus/D. farinae, cat/dog. This underlines the importance of a careful judgement when drawing clinical conclusions and giving the patient guidance based on SPT results. However, clinical relevance of sensitizations is sometimes difficult to determine. In this study, a rigid patient history was used, but patients may not have been aware of exposure to some of the allergens. This holds particularly true for blatella where the poorest correlation was found. Patients may not have realized exposure to household dust, for example, on vacations.
Whether a positive SPT in an asymptomatic subject indicates a future risk of developing allergy is largely unknown as studies are rare. Asymptomatic skin sensitization is a common condition affecting 8–30% of the population when using a local standard panel of aeroallergens 24. Prospective studies show that 30–60% become allergic, depending on allergens and follow-up period. College students were followed up to 23 years, and the risk of rhinitis was more than two times greater in asymptomatic SPT-positive than in SPT-negative subjects 11. Sometimes reassessment of clinically insignificant positive results is warranted, especially if testing has been carried out in winter. Patients may also have overlooked mild symptoms during the previous season which might be apparent in the upcoming season.
Study limitations
In this 17-centre study, we cannot be absolutely sure that all the clinical interpretations were uniform, when the numerous clinicians explored the clinical relevance of positive SPT results. All the participants used, however, the same standard operational procedure (SOP), attached as a supplemental file. For practical reasons, clinical relevance was asked only, if wheal sizes were 3 mm or larger. Patients often report symptoms even though SPT is negative. The association of these symptoms with increased production of serum IgE has not been systematically studied but is questionable.
Inhalant allergens from certain manufacturers were used, and the result may not be generalizable even to same allergens from other manufacturers. Manufacturers have standard procedures to check and label the immunochemical and biological potency of their allergen products, but the potency of allergens may differ between manufacturers. That is why the present results are indicative, but not conclusive in the various settings of practicing allergists. Furthermore, the recombinant allergens and allergen components are increasingly developed, and their clinical relevance remains to be studied in larger patient sets.
Conclusion
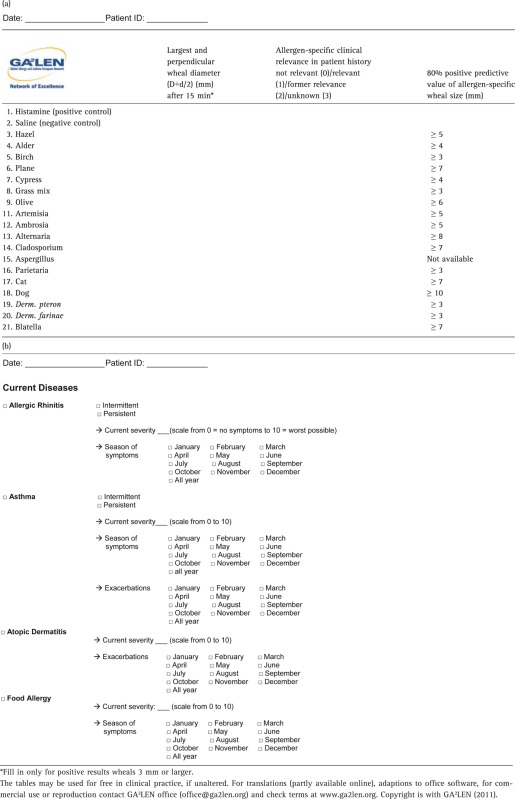
The present analyses draw attention to the necessity skilful interpretation of SPT results to improve testing quality and to avoid medicalization. The SPT key readings can be used in practitioners′ daily work to better interpret SPT results. Their precision, however, depends on adherence to the SPT procedures employed in this GA2LEN study 8 with 1) use of standardized allergens, 2) use of appropriate lancets, and 3) assessing the test (largest wheal diameter) after 15 min. A diagnostic sheet (Table2) for clinical work to copy or download (http://www.ga2len.org), when using the GA²LEN standard allergen panel, is given with the present article.
Table 2.
Official GA2LEN (EU Allergy network): (a) skin prick test documentation form for inhalant allergens, (b) diagnostic sheet for allergic conditions
 |
Conflict of Interest
T. Haahtela has been on the advisory board of Boehringer-Ingelheim, MSD and Orion Pharma and has received payment for lectures from ALK-Abello, Astellas Pharma, MSD and Orion Pharma. J. Bousquet has received honoraria for participation in scientific and advisory boards, giving lectures, and press engagements for Actelion, Almirall, AstraZeneca, Chiesi, GlaxoSmithKline, Meda, Merck, Merck Sharpe & Dohme, Novartis, oM Pharma, Sanofi-Aventis, Schering Plough, Stallergènes, Takeda, Teva and Uriach. U. Darsow has been speaker, investigator and/or been a member of advisory boards for Allergopharma, ALK Abello′, Bencard, GSK, Hermal, MEDA, Novartis Pharma, Stallergenes, Stiefel. The department of Wytske Fokkens has received financial aid for studies from GSK, Bioinspire and Stallargens. She has also been active in advisory boards for J&J and Stallargens and had travel aid as invited speaker from GSK and MSD. N. G. Papadopoulos has received payment for consultancy work from ABBOTT, Novartis, Menarini, for lectures from MSD, URIACH, GSK, ALLERGOPHARMA, Stallergens, MEDA for development of educational presentations from MSD, URIACH, MEDA and grants from Nestle, GSK, MSD.
Supporting Information
Additional Supporting Information may be found in the online version of this article:
Pan-European skin prick test project (PEP).
Protocol for Pan-European Skin prick test.
References
- Demoly P, Romano A, Bousquet J. In vivo methods for the study of allergy. In: Adkinson NF, Yunginger JW, Busse WW, Bochner BS, Simons FER, Holgate ST, editors. Middleton's allergy, principles and practice. 7th edn. Philadelphia, PA: Elsevier Inc; 2008. pp. 1267–80. [Google Scholar]
- Burbach GJ, Heinzerling LM, Edenharter G, et al. GA2LEN skin test study II: clinical relevance of inhalant allergen sensitizations in Europe. Allergy. 2009;64:1507–15. doi: 10.1111/j.1398-9995.2009.02089.x. [DOI] [PubMed] [Google Scholar]
- Bousquet J, Khaltaev N. Global surveillance, prevention and control of chronic respiratory diseases. A comprehensive approach. Global alliance against chronic respiratory diseases. Geneva: World Health Organization; ISBN 978 92 4 156346 8 2007:148. [Google Scholar]
- Bock S, Buckley J, Holst A, May C. Proper use of skin tests with food extracts in diagnosis of food hypersensitivity. Clin Allergy. 1978;8:559–64. doi: 10.1111/j.1365-2222.1978.tb01509.x. [DOI] [PubMed] [Google Scholar]
- Sampson HA. Utility of food-specific IgE concentrations in predicting symptomatic food allergy. J Allergy Clin Immunol. 2001;107:891–6. doi: 10.1067/mai.2001.114708. [DOI] [PubMed] [Google Scholar]
- Bousquet J, Burney PG, Zuberbier T, et al. GA2LEN (Global Allergy and Asthma European Network) address the allergy and asthma ‘epidemic’. Allergy. 2009;64:969–77. doi: 10.1111/j.1398-9995.2009.02059.x. [DOI] [PubMed] [Google Scholar]
- Heinzerling L, Frew AJ, Bindslev-Jensen C, et al. Standard skin prick testing and sensitization to inhalant allerges across Europe – a survey from the GALEN Network. Allergy. 2005;60:1287–300. doi: 10.1111/j.1398-9995.2005.00895.x. [DOI] [PubMed] [Google Scholar]
- Heinzerling L, Burbach GJ, Edenharter G, et al. GA2LEN skin test study I: GA2LEN harmonization of skin prick testing: novel sensitization patterns for inhalant allergens in Europe. Allergy. 2009;64:1498–506. doi: 10.1111/j.1398-9995.2009.02093.x. [DOI] [PubMed] [Google Scholar]
- Heinzerling L, Mari A, Bergmann KC, et al. The skin prick test - European standards. Clin Transl Allergy. 2013;3:3. doi: 10.1186/2045-7022-3-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bousquet P-J, Burbach G, Heinzerling LM, et al. GA2LEN skin test study III: Minimum battery of test inhalant allergens needed in epidemiological studies in patients. Allergy. 2009;64:1656–62. doi: 10.1111/j.1398-9995.2009.02169.x. [DOI] [PubMed] [Google Scholar]
- Settipane RJ, Hagy GW, Settipane GA. Long-term risk factors for developing asthma and allergic rhinitis: a 23-year follow-up study of college students. Allergy Asthma Proc. 1994;15:21–5. doi: 10.2500/108854194778816634. [DOI] [PubMed] [Google Scholar]
- Cydulka RK, Emerman CL, Rowe BH, et al. MARC Investigators. Difference between men and women in reporting of symptoms during an asthma exacerbation. Ann Emerg Med. 2001;38:123–8. doi: 10.1067/mem.2001.114305. [DOI] [PubMed] [Google Scholar]
- Dreborg S. Allergy. 1989;10:1–59. Skin tests used in type I allergy testing. Position paper. [Google Scholar]
- Rhodius R, Wickens K, Cheng S, Crane J. A comparison of two skin test methodologies and allergens from two different manufacturers. Ann Allergy Asthma Immunol. 2002;88:374–9. doi: 10.1016/S1081-1206(10)62367-8. [DOI] [PubMed] [Google Scholar]
- Rueff F, Przybilla B, Bilo MB, et al. Predictors of severe systemic anaphylactic reactions in patients with Hymenoptera venom allergy: importance of baseline serum tryptase-a study of the European Academy of Allergology and Clinical Immunology Interest Group on Insect Venom Hypersensitivity. J Allergy Clin Immunol. 2009;124:1047–54. doi: 10.1016/j.jaci.2009.08.027. [DOI] [PubMed] [Google Scholar]
- Bernstein IL, Storms WW. Practice parameters for allergy diagnostic vtesting. Joint Task Force on Practice Parameters for the Diagnosis and Treatment of Asthma. The American Academy of Allergy, Asthma and Immunology and the American College of Allergy, Asthma and Immunology. Ann Allergy Asthma Immunol. 1995;2:543–625. [PubMed] [Google Scholar]
- Vocks E, Stander K, Rakoski J, Ring J. Suppression of immediate-type hypersensitivity elicitation in the skin prick test by ultraviolet B irradiation. Photodermatol Photoimmunol Photomed. 1999;15:236–40. doi: 10.1111/j.1600-0781.1999.tb00096.x. [DOI] [PubMed] [Google Scholar]
- Rueff F, Bergmann KC, Brockow K, et al. Skin tests for diagnostics of allergic immediate-type reactions. Guideline of the German Society for Allergology and Clinical Immunology. Pneumologie. 2011;65:484–95. doi: 10.1055/s-0030-1256476. [DOI] [PubMed] [Google Scholar]
- Nelson HS, Knoetzer J, Bucher B. Effect of distance between sites and region of the body on results of skin prick tests. J Allergy Clin Immunol. 1996;97:596–601. doi: 10.1016/s0091-6749(96)70304-4. [DOI] [PubMed] [Google Scholar]
- Carr WW, Martin B, Howard RS, Cox L, Borish L. Comparison of test devices for skin prick testing. J Allergy Clin Immunol. 2005;116:341–6. doi: 10.1016/j.jaci.2005.03.035. [DOI] [PubMed] [Google Scholar]
- Position paper: allergen standardization and skin tests. The European Academy of Allergology and Clinical Immunology. Allergy. 1993;48(Suppl. 14):48–82. [PubMed] [Google Scholar]
- Bousquet PJ, Chatzi L, Jarvis D, Burney P. Assessing skin prick tests reliability in ECRHS-I. Allergy. 2008;63:341–6. doi: 10.1111/j.1398-9995.2007.01581.x. [DOI] [PubMed] [Google Scholar]
- Ahlstedt S. Understanding the usefulness of specific IgE blood tests in allergy. Clin Exp Allergy. 2002;32:11–6. doi: 10.1046/j.0022-0477.2001.01289.x. [DOI] [PubMed] [Google Scholar]
- Bodtger U. Prognostic value of asymptomatic skin sensitization to aeroallergens. Curr Opin Allergy Clin Immunol. 2004;4:5–10. doi: 10.1097/00130832-200402000-00003. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Pan-European skin prick test project (PEP).
Protocol for Pan-European Skin prick test.