Abstract
Background and Objective: Laser vaporization of the prostate is considered to be a promising treatment for benign prostatic hyperplasia (BPH), and efficiency of vaporization and hemostasis are both important parameters for such treatment. In this study, we used a high-power laser diode with a wavelength of 980 nm to obtain high vaporization efficiency with good hemostasis. The objective of this study is to evaluate the efficacy of laser vaporization for treatment of BPH in ex vivo experiments using a 300-W high-power laser diode with a wavelength of 980 nm quantitatively.
Materials and Methods: An ex vivo experimental setup simulating clinical treatment situation was constructed. Bovine prostate tissue was used as a sample. The power setting was 100, 150, 200, 250, or 300 W, and the irradiation time was 0.5, 1, or 2 s. After laser irradiation, vaporized and coagulated depths were measured.
Results: The vaporized depth increased with the laser power and irradiation time, and the results confirmed that the high-power laser diode could efficiently vaporize the prostate tissue. Coagulated depth increased as the laser power became higher.
Conclusions: Laser vaporization of prostate tissue using a high-power laser diode with a wavelength of 980 nm represents a promising treatment for BPH; this method exhibits high vaporization efficiency and good hemostasis. However, operators must be aware of the risk of postoperative perforation of the prostatic capsule caused by coagulation of deep regions that cannot be visualized by endoscopic observation.
Keywords: absorption coefficient, laser surgery, scattering coefficient, transurethral vaporization of the prostate
Introduction
The lasers, such as the holmium:yttrium aluminum garnet (Ho:YAG) laser at a wavelength of 2140 nm and the frequency-doubled Nd:YAG (potassium titanyl phosphate [KTP]) laser at a wavelength of 532 nm have been used most commonly for benign prostatic hyperplasia (BPH) instead of transurethral resection of the prostate (TURP), which is likely to cause bleeding and transurethral resection sydrome. 1–5) Although the Ho:YAG laser is superior in terms of the cutting capability due to the high absorption coefficient of water and holmium laser enucleation of the prostate (HoLEP) is a useful treatment, HoLEP requires the operator to be highly skilled. 6–7) By contrast, the KTP laser is delivered transparently through water but is strongly absorbed by hemoglobin. Thus, laser vaporization of the prostate by KTP laser can be performed with good hemostasis, but it requires a long operating duration. 3,4,8,9) In recent years, a 980-nm laser diode has been applied to laser vaporization of the prostate. 5,9,10) This wavelength offers high simultaneous absorption by water and hemoglobin, so it combines efficient vaporization of the prostate with good hemostasis. 9,10) In addition, the resultant increase in vaporization efficiency shortens the duration of the operation.
Although the coagulation induced around the vaporized area is useful for hemostasis, the areas damaged by coagulation and necrosis cause irritative events and perforation postoperatively. 11,12) Therefore, it is necessary to quantitatively evaluate not only the vaporization depth but also the depths of coagulation and necrosis.
A high-power laser diode at a wavelength of 980 nm and a output power of 300 W has the potential to treat BPH safely and effectively. However, the interactions between laser light and prostate tissue have never been quantitatively measured. Therefore, in this study, we constructed an ex vivo experimental setup simulating a clinical treatment situation, and quantitatively evaluated the safety and efficacy of laser vaporization of the prostate using a high-power laser diode at a wavelength of 980 nm.
Materials and Methods
Measurement of optical properties
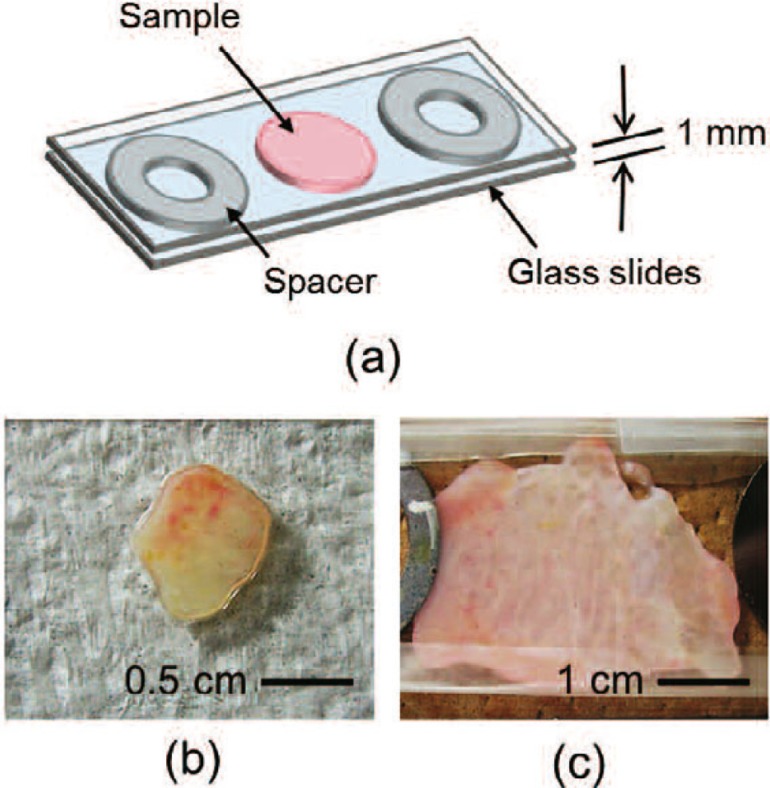
Because it is difficult to obtain a regular source of human prostate tissue, we used bovine prostate tissue to evaluate the effects of laser irradiation. To validate this model, we first measured the optical properties of bovine prostate. The human prostate tissue was extracted from a patient for treatment of BPH by TURP. Then, the prostate tissue was kept in saline at 4°C for 16 days. The bovine prostate tissues were obtained from cattle originally slaughtered to obtain meat. The prostate tissues were immediately delivered in cold storage and were subsequently kept in saline at 4°C for 2 days. It should be noted that the human prostate tissue was not extracted for this study and we also obtained the patient's consent about the aim of this study. Similarly, the cattle was not sacrificed for this study. The prostate tissues were sliced to a thickness of about 1 mm, and placed in a sample holder made from two glass slides and spacers with a thickness of 1 mm. Fig. 1 shows a schematic of a sliced tissue placed in a sample holder and photographs of human and bovine prostate tissues used for the measurement of optical properties. The photographs of (b) and (c) are a part of a sample placed in a sample holder from two glass slides after slicing to a thickness of about 1 mm.
Fig. 1:
(a) Schematic of a sliced tissue placed in a sample holder and photographs of (b) human (c) bovine prostate-tissue samples used for the measurement of optical properties.
The optical properties were measured using a double-integrating-sphere optical system and the inverse Monte Carlo method. 13,14) The double-integrating-sphere optical system was used to measure the diffuse reflectance Rd and the total transmittance Tt of the tissues, taking optical scattering into account. The sample was placed between two integrating spheres (CSTM-3P-GPS-033SL, Labsphere, Inc., USA) and irradiated with white light from a xenon lamp (L2274[GS], Hamamatsu Photonics K.K., Japan). The first sphere was used to collect the diffusely reflected light, whereas the second sphere was used to collect the transmitted light. The light from each integrating sphere was transmitted through an optical fiber (CUSTOM-PATCH-2243142, Ocean Optics, Inc., USA) to a spectrometer (Maya2000-Pro, Ocean Optics), and measured with an exposure time of 100 ms. From the measured values Rd and Tt, the set of optical properties of the tissue, i.e., the absorption coefficient µa and scattering coefficient µs, were calculated using the inverse Monte Carlo method. 13,14) We used Monte Carlo calculation code obtained from the website of the Oregon Medical Laser Center at Providence St. Vincent Medical Center. 15) The reduced scattering coefficient µs′ was calculated by
where g is the anisotropy factor of scattering, here set to 0.862. 16) Optical penetration depth δ was calculated by
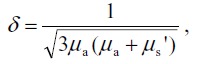 |
where δ is the depth at which the power density decreases to 1/e of its initial value at the surface. 17)
Ex vivo experimental setup for laser irradiation
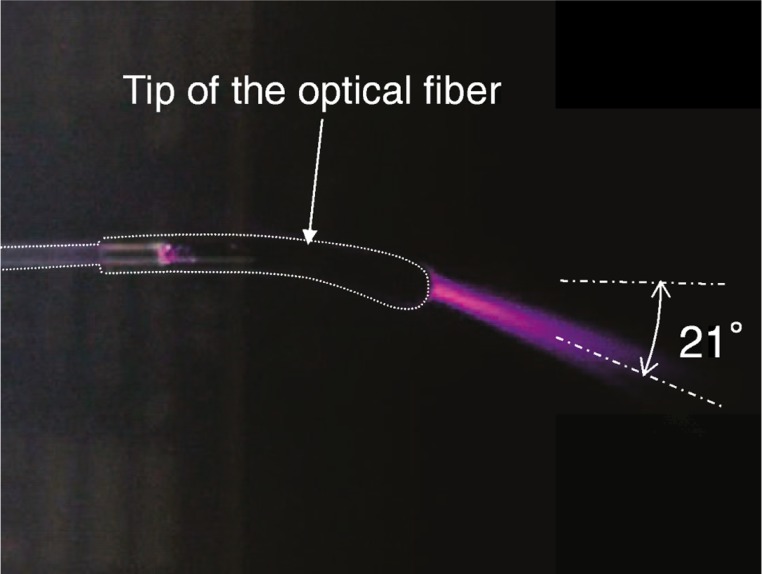
Laser irradiation was performed using a 300-W high-power laser diode at a wavelength of 980 nm (Ceralas HPD 300, Biolitec AG, Germany) and an optical fiber with a curved tip (Twister™ Large Fiber, Biolitec). 18,19) The core diameter and the length of the optical fiber were 600 µm and 3 m 19), respectively. Fig. 2 shows a photograph of the laser light emitted from the optical fiber and scattered in an aqueous suspension of lipid droplets. Laser light was emitted in a direction about 21° from the axis of the optical fiber. The output laser power emitted from the tip of the fiber was calibrated using a power meter (FL400A-BB-50, Ophir Optronics Solutions Ltd., Israel).
Fig. 2:
Photograph of laser light emitted from the optical fiber and scattered in an aqueous suspension of lipid droplets.
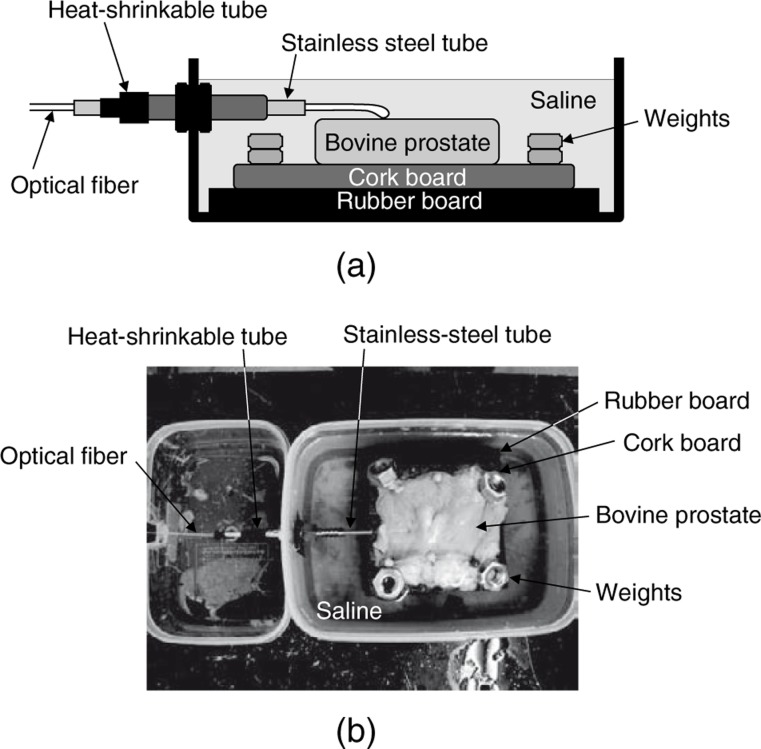
Fig. 3 shows the ex vivo experimental setup. The clinical treatment of BPH is performed using a rigid endoscope under circulating saline, and laser irradiation is performed by placing the tip of the optical fiber in contact with the surface of the prostate. Similarly, in this ex vivo experimental setup, laser irradiation was performed in saline by placing the tip of the optical fiber in contact with the surface of the prostate sample, and a stainless-steel tube was used to simulate the working channel of the rigid endoscope. An optical fiber with a maximum diameter of 1.85 mm was inserted through a stainless-steel tube. The inner diameter and length of this stainless-steel tube were 3.19 and 115 mm, respectively. The plastic case was filled with saline, and the stainless-steel tube and the joint were covered with a heat-shrinkable tube to prevent leakage of saline. A segment of bovine prostate tissue was put on a cork board and immersed in saline. The height of the surface of the prostate tissue was adjusted by placing rubber boards under the cork board. In order to minimize the variation from sample to sample, all samples of bovine prostate tissue were delivered and kept in the same way as those used for measurement of the optical properties. The size of the sample was about 40×60 mm2. The laser irradiation was performed in continuous-wave mode; the power setting was 100, 150, 200, 250, or 300 W, and the irradiation time was 0.5, 1, or 2 s. In this study, the fiber tip was in contact with the surface of the prostate tissues during laser irradiation. Therefore, the spot size for laser irradiation was almost same as the core diameter of 600 µm.
Fig. 3:
(a) Schematic and (b) photograph of the ex vivo experimental setup for contact laser irradiation.
In the clinical treatment of BPH by the laser system and optical fiber used in this study, the tip of the optical fiber must in contact with the surface of the prostate tissue and must continuously be moved by manual operation of the doctor. Therefore, it is expected that the irradiation time for a point on the prostate tissue is less than 1 s. Thus, the irradiation times of 0.5 and 1 s were set as the normal irradiation time, and the irradiation time of 2 s was set under the assumption of excessive irradiation condition.
After laser irradiation, the prostate tissue was cut at the center of the irradiation point, vertically to the surface, and the cross-section was observed to measure the vaporized and coagulated depths. The irradiated prostate tissues were fixed with a histological fixative (Mildform® 20NM, Wako Pure Chemical Industries, Ltd., Japan) and stained with hematoxylin and eosin. Histological analysis was performed by Histo Science Laboratory Co., Ltd. (Japan).
Results and Discussion
Optical properties of human and bovine prostate tissues
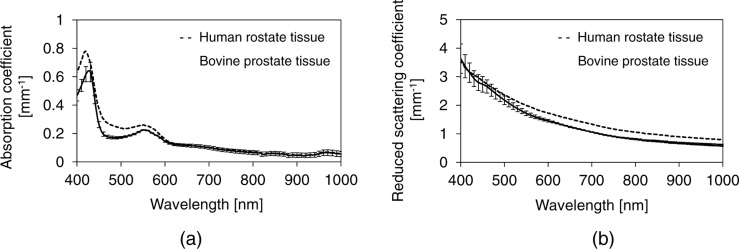
Fig. 4 shows the measured absorption and scattering spectra of both human and bovine prostate tissues, respectively. Table 1 shows the measured optical properties of human and bovine prostate tissues at a wavelength of 980 nm. The absorption coefficient µa and the reduced scattering coefficient µs′ of human prostate tissue were similar to those of the bovine prostate tissue. Thus, the optical penetration depth δ of the human prostate tissue was also similar to that of the bovine prostate tissue. These results suggest that bovine prostate tissue is a valid model for human prostate tissue for evaluation of the effects of laser irradiation.
Fig. 4:
The measured (a) absorption and (b) reduced scattering coefficient spectra of the human and bovine prostate tissues in at the wavelength of 350–1000 nm.
Table 1: Absorption coefficient µa, reduced scattering coefficient µs′, and optical penetration depth δ of the prostate tissues at a wavelength of 980 nm, measured using a double-integrating-sphere optical system and the inverse Monte Carlo method. n indicates the number of samples.
| µa [mm−1] | µs′ [mm−1] | δ [mm] | |
|---|---|---|---|
| Human prostate tissue (n = 1) | 0.066 | 0.81 | 2.4 |
| Bovine prostate tissue (n = 3) | 0.061 ± 0.021 | 0.61 ± 0.04 | 2.9 ± 0.5 |
The sliced tissues might be different from the living tissue in the amount of blood. Since all samples were stored in saline, blood might flow away from the samples. As shown in Fig. 4, the difference in the height of absorption peaks of the human and bovine prostate tissues at the wavelength of about 420 and 550 nm are caused by the difference in the amount of blood. However, the effect of the difference in the amount of blood is negligible at the wavelength of 980 nm as shown in Fig. 4.
Vaporized and coagulated depths
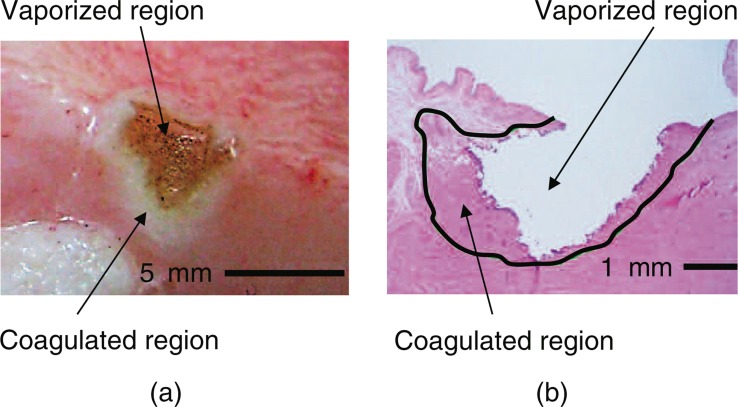
Fig. 5 shows a typical photograph of the surface and a typical histological image of bovine prostate tissue after laser irradiation. The histological image reveals that the surface of the vaporized region was slightly carbonized and surrounded by a coagulated region, although the extent of carbonization was negligible.
Fig. 5:
(a) Typical photograph of the surface and (b) typical histological image of bovine prostate tissue following laser irradiation with a laser power setting of 200 W and an irradiation time of 1 s. The histological sample was stained with hematoxylin and eosin.
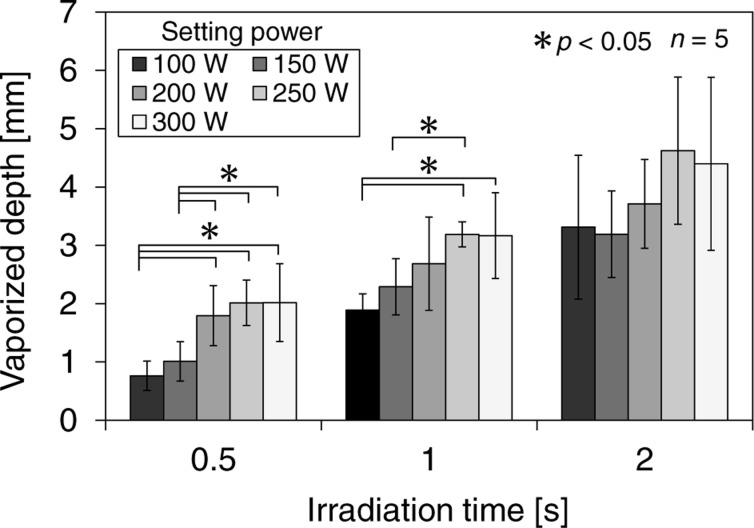
Fig. 6 shows the relationship between the vaporized depth and the power setting for each irradiation time. The vaporized depth increased with laser power and irradiation time. Statistical comparison was made using the t-test, and differences were considered to be significant at p < 0.05. Significant differences were observed between power setting of 100 and 300 W for the irradiation time of 0.5 and 1 s. Therefore, the 300-W high-power laser diode at 980 nm can efficiently vaporize the prostate tissue for the short irradiation time and contribute to a reduction in the duration of the operation. In the irradiation time of 2 s, the deviation of the vaporized depth among samples at all setting power was large, so no significant differences were observed. The optical penetration depth of bovine prostate tissue shown in table 1 was 2.9 ± 0.5 mm at a wavelength of 980 nm. Although the samples were prepared on the same protocol, the variation from sample to sample might involve the deviation of the vaporized depth in view of the deviation of the optical penetration depth.
Fig. 6:
Relationship between the vaporized depth and the laser power setting for each irradiation time. n indicates the number of the samples. Data are expressed as means, and the error bars are the standard deviations of the mean.
The photothermal effect induced by laser irradiation was used for removal of prostate tissue in this treatment method. When laser light is irradiated to the prostate tissue, the rapid increase in the local temperature of the prostate tissue leads to the rapid increase in the local pressure due to the vaporization of the tissue, and the section of the tissue is removed by microexplosion of the tissue 20).
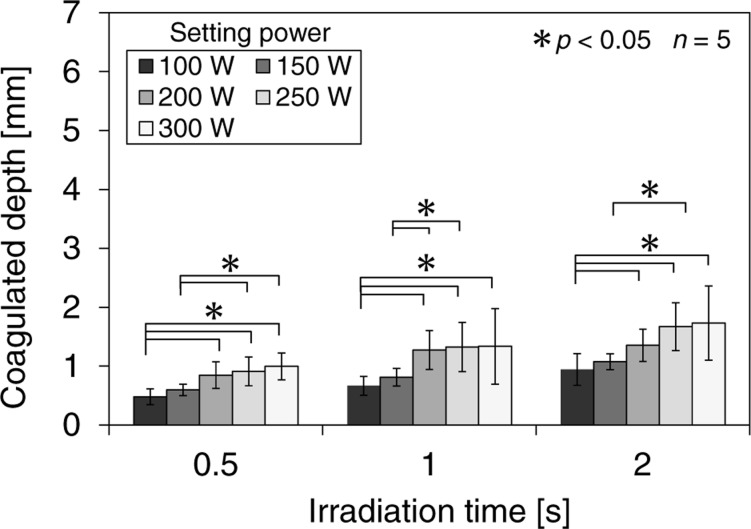
Fig. 7 shows the relationship between the coagulated depth and the power setting for each irradiation time. The coagulated depth also increased with laser power and irradiation time. However, the change in the coagulated depth was relatively smaller than the change in the vaporized depth. Although coagulation is useful for hemostasis, 21) excessively deep coagulation can irritate the urethra and lead to postoperative perforation. 12) Therefore, excess coagulation should be avoided. The results shown in Figs. 6 and 7 indicate that vaporization using the 300-W high-power laser results in minimal coagulation as well as a high vaporization rate. Operators can use endoscopic observation to recognize and control the extent of vaporization; however, endoscopy cannot reveal the extent of coagulation during the operation. To prevent postoperative perforation, operators need to understand the relationship between the coagulated depth and the laser-irradiation conditions, and be aware of the risk of postoperative perforation of the prostatic capsule.
Fig. 7:
Relationship between the coagulated depth and the laser power setting for each irradiation time. n indicates the number of the samples. Data are expressed as means, and the error bars are the standard deviations of the mean.
Irradiation effects by a laser diode with the wavelength of 980 nm have been investigated in previous preclinical and clinical studies. A preclinical study using a 150-W laser diode with 980 nm showed that deep coagulation caused by laser irradiation led to prostatic slough-induced obstruction and perforation in six of eight surviving animals. 22) On the other hand, a previous clinical study suggested that the clinical outcomes by a 300-W laser diode with 980 nm were good. Considering the results of these studies, the laser-tissue interaction should be evaluated quantitatively irrespective of operator's skill. Therefore, ex vivo evaluation using a 300-W laser diode with 980 nm was conducted in this study.
In a previous ex vivo experiment using porcine kidneys at a laser power of 80 W, the vaporization rate at 980 nm was higher than the rate at 532 nm using a KTP laser, whereas the hemostatic properties of the two wavelengths were equivalent. 9) The results of this study showed that the coagulated depth increased with laser power and irradiation time. Considering coagulation is useful for preventing bleeding, the 300-W high-power laser diode at a wavelength of 980 nm contributes to the treatment with good hemostasis. 9) Therefore, the 300-W high-power laser diode at a wavelength of 980 nm should be useful for treatment of BPH with high vaporization rate and good hemostasis. However, this study did not evaluate the hemostatic properties directly, so additional in vivo study or ex vivo study are needed for precise evaluation of the hemostatic properties.
This study evaluated the efficacy of laser vaporization using the 300-W high-power laser diode. As a similar study, a study using a high-power laser diode at a wavelength of 980 nm has already been performed. 24) This previous study conducted an ex vivo experiment using a 200-W high-power laser diode with 980 nm and showed that the vaporized volume increased with laser power. Although this previous study suggested a relationship between power setting and vaporization similar to this study, it is different from this study in the irradiation method between this study and the previous study, such as the shape of the optical fibers and the contact laser irradiation. The optical fiber used in this study has a unique shape and laser light is emitted in a direction about 21° from the axis of the optical fiber. It is designed for laser irradiation in a contact mode, so it is easy to contact the prostate tissue and can achieve laser irradiation with high energy density relative to the side firing fiber. 18) The high power density at the tip causes instant tissue vaporization with a minimal coagulative layer beneath the ablated surface. 18)
Conclusion
In this study, we quantitatively evaluated the effects of laser vaporization of the prostate using a 300-W high-power laser diode at a wavelength of 980 nm, in an ex vivo experiment simulating the clinical situation. The results of the ex vivo experiments showed that vaporized and coagulated depths increased with laser power setting and irradiation time. However, the change in the coagulated depth was relatively smaller than the change in the vaporized depth. These results suggest that laser vaporization of the prostate using a 300-W high-power laser diode at a wavelength of 980 nm represents a potentially effective treatment for BPH with high vaporization rate and good hemostasis.
Acknowledgements
The authors thank Reiko Ikahata, Hiromi Sekizaki, and Takashi Kuroda of Integral Corp. for their technical support.
References
- 1: Marszalek M, Ponholzer A, Pusman M, Berger I, Madersbacher S. (2009): Transurethral resection of the prostate. European Urology Supplements, 8:504 - 512 [Google Scholar]
- 2: Madersbacher S, Marberger M. (1999): Is transurethral resection of the prostate still justified? BJU International, 83:227 - 237 [DOI] [PubMed] [Google Scholar]
- 3: Kuntz RM. (2006): Current role of lasers in the treatment of benign prostatic hyperplasia (BPH). European Urology, 49:961 - 969 [DOI] [PubMed] [Google Scholar]
- 4: Marks AJ, Teichman JMH. (2007): Lasers in clinical urology: state of the art and new horizons. World Journal of Urology, 25:227 - 233 [DOI] [PubMed] [Google Scholar]
- 5: Gravas S, Bachmann A, Reich O, Roehrborn CG, Gilling PJ, Rosette JDL. (2011): Critical review of lasers in benign prostatic hyperplasia (BPH). BJU International, 107:1030 - 1043 [DOI] [PubMed] [Google Scholar]
- 6: Gilling P. (2008): Holmium laser enucleation of the prostate (HoLEP). BJU International, 101:131 - 142 [DOI] [PubMed] [Google Scholar]
- 7: Elzayat EA, Elhilali MM. (2007): Holmium laser enucleation of the prostate (HoLEP): long-term results, reoperation rate, and possible impact of the learning curve. European Urology, 52:1465 - 1472 [DOI] [PubMed] [Google Scholar]
- 8: Reich O, Bachmann A, Schneede P, Zaak D, Sulser T, Hofstetter A. (2004): Experimental comparison of high power (80 W) potassium titanyl phosphate laser vaporization and transurethral resection of the prostate. Journal of Urology, 171:2502 - 2504 [DOI] [PubMed] [Google Scholar]
- 9: Wendt-Nordahl G, Huckele S, Honeck P, Alken P, Knoll T, Michel MS, Hacker A. (2007): 980-nm diode laser: a novel laser technology for vaporization of the prostate. European Urology, 52:1723 - 1728 [DOI] [PubMed] [Google Scholar]
- 10: Leonardi R, Caltabiano R, Lanzafame S. (2010): Histological evaluation of prostatic tissue following transurethral laser resection (TULaR) using the 980 nm diode laser. Archivio Italiano di Urologia e Andrologia, 82:1 - 4 [PubMed] [Google Scholar]
- 11: Bach T, Muschter R, Sroka R, Gravas S, Skolarikos A, Herrmann TR, Bayer T, Knoll T, Abbou CC, Janetschek G, Bachmann A, Rassweiler JJ. (2012): Laser treatment of benign prostatic obstruction: basics and physical differences. European Urology, 61:317 - 325 [DOI] [PubMed] [Google Scholar]
- 12: Chen CH, Chiang PH, Chuang YC, Lee WC, Chen YT, Lee WC. (2010): Preliminary results of prostate vaporization in the treatment of benign prostatic hyperplasia by using a 200-W high-intensity diode laser. Urology, 75:658 - 663 [DOI] [PubMed] [Google Scholar]
- 13: Honda N, Ishii K, Awazu K. (2012): Optical properties measurement of the laser-ablated tissues for the combined laser ablation with photodynamic therapy. Proceeding SPIE, 8221:82210F [Google Scholar]
- 14: Honda N, Kariyama Y, Ishii T, Abe C, Inoue K, Ishizuka M, Tanaka T, Hazama H, Awazu K. (2013): Optical properties of tumor tissues grown on the chorioallantoic membrane of chicken eggs measured with a double integrating sphere and inverse Monte Carlo method in the wavelength range of 350–1000 nm. Proceeding SPIE, 8579:85790T [Google Scholar]
- 15: Oregon Medical Laser Center at Providence St. Vincent Medical Center. http://omlc.ogi.edu/software/
- 16: Methods and Algorithms for the Measurement of the Optical Parameters of Tissues. In: (Tuchin V, ed.) Tissue Optics: light scattering methods and instruments for medical diagnosis. 2007, SPIE Press, Bellingham. pp 164 [Google Scholar]
- 17: Optical Properties of Tissues with Strong (Multiple) Scattering. In: (Tuchin V, ed.) Tissue Optics: light scattering methods and instruments for medical diagnosis. 2007, SPIE Press, Bellingham. pp 13 [Google Scholar]
- 18: Shaker HS, Shoeb MS, Yassin MM, Shaker SH. (2012): Quartz head contact laser fiber: a novel fiber for laser ablation of the prostate using the 980 nm high power diode laser. Journal of Urology, 187:575 - 579 [DOI] [PubMed] [Google Scholar]
- 19: Shaker H, Alokda A, Mahmoud H. (2012): The Twister laser fiber degradation and tissue ablation capability during 980-nm high-power diode laser ablation of the prostate. A randomized study versus the standard side-firing fiber. Lasers in Medical Science, 27:959 - 963 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20: Interaction Mechanisms. In: (Niemz M H.) Laser-Tissue Interactions: Fundamentals and Applications. 2004, Springer, New York. pp 45-150 [Google Scholar]
- 21: Wezel F, Wendt-Nordahl G, Huck N. (2010): New alternatives for laser vaporization of the prostate: experimental evaluation of a 980-, 1,318- and 1,470-nm diode laser device. World Journal of Urology, 28:181 - 186 [DOI] [PubMed] [Google Scholar]
- 22: Rieken M, Kang HW, Koullick E, Ruth GR, Bachmann A. (2010): Laser vaporization of the prostate in vivo: experience with the 150-W 980-nm diode lasers in living canines. Lasers in Surgery and Medicine, 42:736 - 742 [DOI] [PubMed] [Google Scholar]
- 23: Seitz M, Reich O, Gratzke C, Schlenker B, Karl A, Bader M, Khoder W, Fischer F, Stief C, Sroka R. (2009): High-power diode laser at 980 nm for the treatment of benign prostatic hyperplasia: ex vivo investigations on porcine kidneys and human cadaver prostates. Laser in Medical Science, 24:172 - 178 [DOI] [PubMed] [Google Scholar]