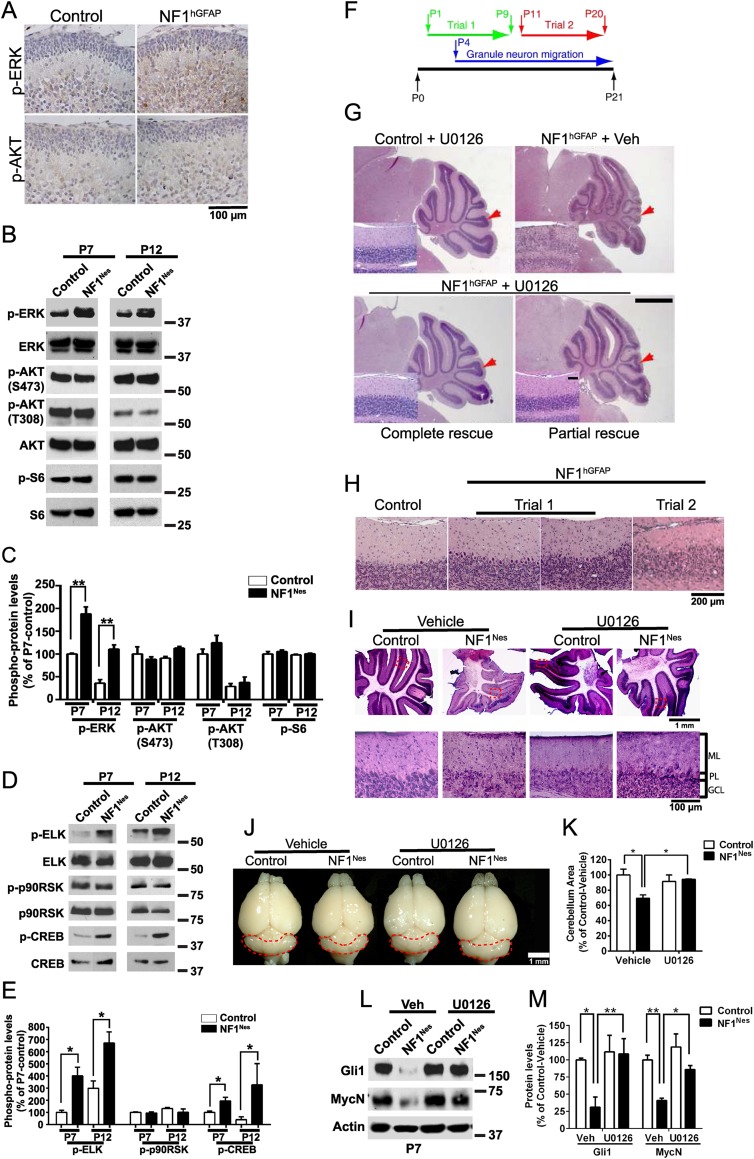
Figure 7.
Postnatal pharmacological inhibition of the ERK pathway prevents cerebellar abnormalities caused by NF1 loss. (A) P2 control and NF1hGFAP cerebella stained with p-ERK and p-AKT (S473) antibodies. (B) Representative Western blot examining ERK and AKT phosphorylation in NF1Nes cerebellum whole lysates. (C) Quantitative analysis indicates elevated p-ERK levels in mutant mice. Mean ± SEM; n = 3; (**) P < 0.01. (D,E) Quantitative Western blot analysis of ERK pathway downstream targets in NF1Nes cerebella. Mean ± SEM; n = 3; (*) P < 0.05. (F) Schematic of the U0126 administration protocols used for treatment of control and mutant animals. Trial 1 was performed perinatally, while trial 2 was performed after GNPs had completed proliferative expansion. (G) Cerebellar morphology analysis by H&E staining in control and NF1hGFAP mice subjected to trial 1 of U0126 administration. Control mice treated with U0126 or vehicle showed similar cerebellar gross morphology. Red arrows indicate folium VII. Insets show folium VII layering. Bar: 1 mm; insets, 100 μm. (H) Cerebellar structural defects in NF1hGFAP mice subjected to trial 2 of U0126 administration. (I–K) Trial 1 paradigm U0126 administration ameliorates folia layering abnormalities in NF1Nes cerebellum. The bottom panels show high-magnification images of regions encompassed by dashed lines. (J,K) Analysis of overall cerebellar size shows restoration of mutant cerebella by U0126 treatment. Mean ± SEM; n = 3; (*) P < 0.05. (L,M) Quantitative Western blot examination indicates normal Gli1 and MycN protein levels in U0126-treated NF1Nes cerebella at P7. Mean ± SEM; n = 3; (*) P < 0.05; (**) P < 0.01. Molecular weight markers (in kilodaltons) are shown in B, D, and L.