FIGURE 2.
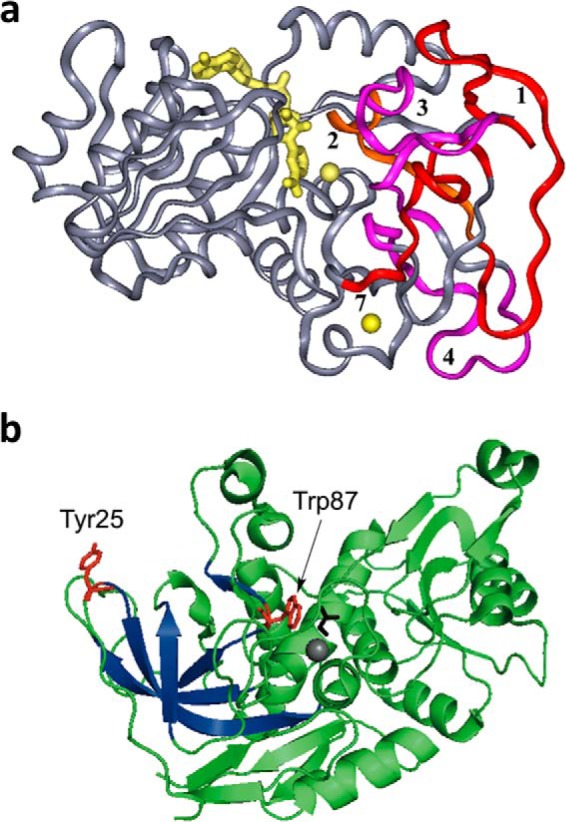
Structural features of ht-ADH. a, the five peptides (1–4 and 7) that increase their flexibility above 30 °C in ht-ADH reside within the substrate-binding site and are colored orange and fuchsia. The cofactor NAD+ has been modeled into the active site and is colored yellow, as are the catalytic zinc ion (near the nicotinamide ring of cofactor) and the structural zinc ion. Reproduced with permission from (37), copyright (2004) National Academy of Sciences, U.S.A. b, the relationship of the surface Tyr-25 (red) to the active site Trp-87 (red). Bound substrate, adjacent to Trp-87, is black. The series of β-sheets that increase their flexibility above 30 °C (see a above) are colored dark blue.
