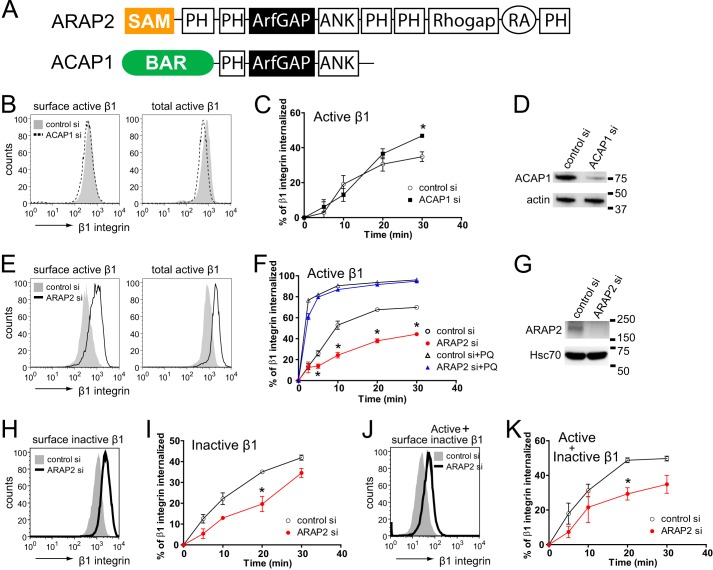
FIGURE 1.
ARAP2 and ACAP1 differentially affect endocytosis and recycling of β1 integrin. A, schematic of the ARAP2 and ACAP1 domain structure. SAM, sterile α-motif; ArfGAP, ArfGTPase-activating protein; Rhogap, RhoGTPase-activating protein; ANK, ankyrin repeat; RA, Ras-association domain; BAR, Bin/Amphiphysin/Rvs. B–K, the net internalization rates of β1 integrin. siRNA-treated HeLa cells were labeled with an anti-active β1 integrin antibody (12G10), an anti-inactive β1 integrin antibody (MAB13), or an antibody that recognizes both the active and inactive forms of β1 integrin (MEM101A) at 4 °C, followed by incubation at 37 °C in the absence or presence of 0.3 mm primaquine (PQ). At each time point, surface β1 integrin was detected using a secondary antibody specific for the anti-β1 integrin antibody and flow cytometry. B, E, H, and J, representative FACS histograms from cells at time 0 (surface integrin) and from permeabilized cells (total integrin). C, F, I, and K, time dependence of integrin internalization. Results are mean ± S.E. from at least three experiments. *, p < 0.05. D and G, the efficiency of ARAP2 and ACAP1 knockdown was determined by immunoblot analyses.