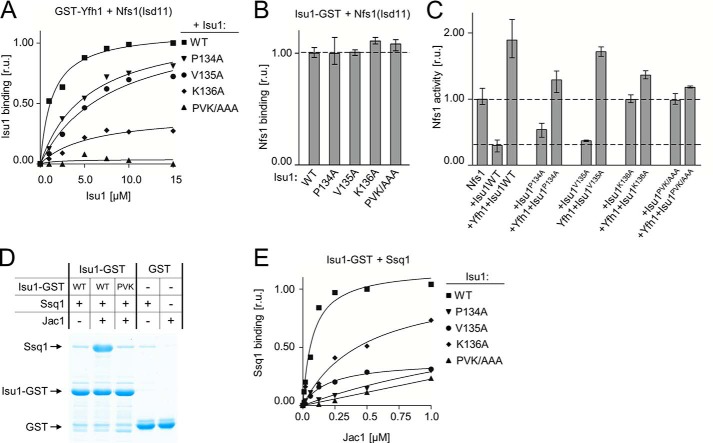
FIGURE 4.
PVK motif of Isu1 is critical for stable Yfh1 interaction with the Isu1-Nfs1(Isd11) complex. A, 2.5 μm GST-Yfh1 and 15 μm Nfs1(Isd11) was mixed with the indicated concentrations of Isu1 (WT) or indicated variant having alanine substitutions in place of Pro134, Val135, and/or Lys136, as indicated. Glutathione resin was added to pull down the GST-Yfh1 and associated proteins, which were separated by SDS-PAGE and stained. Densitometry values plotted as relative units (r.u.) with maximum binding of WT Isu1 protein set at 1. B, 2.5 μm Isu1-GST (WT) or the indicated replacement variant was mixed with 15 μm Nfs1(Isd11). Glutathione resin was added to pull down the GST-Yfh1 and associated proteins, which were separated by SDS-PAGE and stained. Binding of WT was set at 1. Bars represent average value for three independent measurements, with error bars indicating the range of the measurements. C, cysteine desulfurase activity of Nfs1(Isd11) was measured in the absence and presence of the indicated proteins. Activity of Nfs1(Isd11) alone was set to 1. Bars represent average value for three independent measurements, with error bars indicating the range of the measurements. D, 2.5 μm Isu1-GST (WT) or Isu1P134A/V135A/K136A-GST variant (PVK) or GST were incubated in the presence (+) or absence (−) of 4 μm Ssq1 and/or 0.25 μm Jac1. Glutathione resin was added to pull down GST and associated proteins, which were separated by SDS-PAGE and stained. E, 2.5 μm Isu1-GST, WT, or the indicated variants, were mixed with 4 μm Ssq1 and varying concentrations of Jac1. Glutathione resin was added to pull down GST and associated proteins, which were separated by SDS-PAGE and stained. Densitometry values plotted as relative units (r.u.) with maximum binding of Ssq1 set at 1.