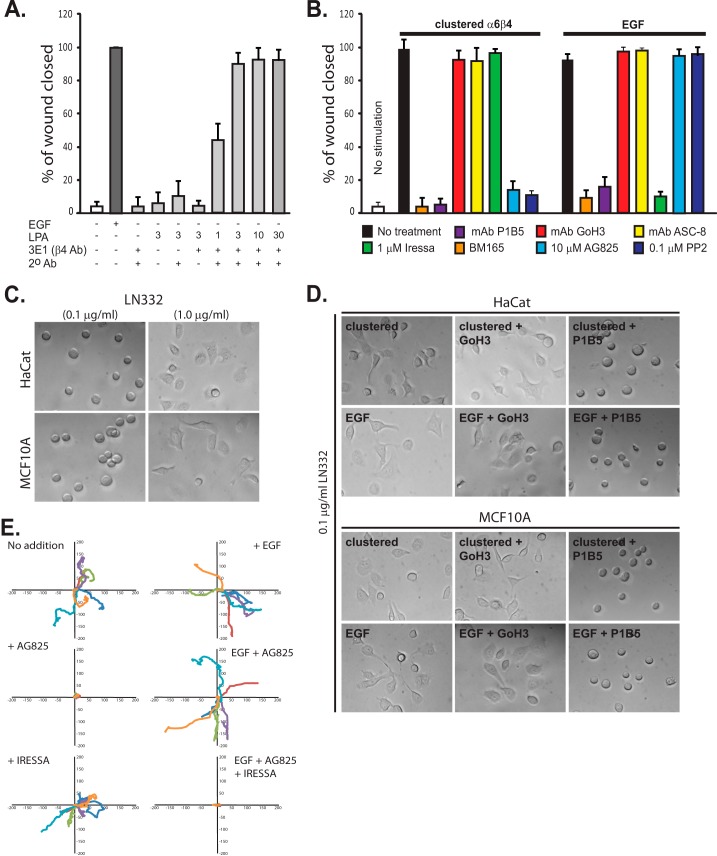
FIGURE 1.
HER2 and EGFR are involved in distinct α6β4-dependent cell motility mechanisms. A, confluent HaCat keratinocytes are starved and then stimulated to migrate for 15–18 h in a scratch wound assay by the addition of 10 ng/ml EGF (EGF chemokinesis) or by clustering of the integrin using mAb3E1 plus a secondary anti-mouse IgG in the presence of 1–30 μm LPA to mimic matrix chemokinesis. Maximal haptotactic migration is observed in the presence of 3 μm LPA, which is used for all remaining experiments. B, HaCat keratinocytes are stimulated to undergo matrix chemokinesis (clustered α6β4) or EGF-stimulated chemokinesis in the presence or absence of mAb P1B5 to block binding by α3β1 integrin, mAb GoH3, or ASC-8 to block α6β4 integrin binding, BM165 to block the integrin-binding sites in LN332, 1 μm Iressa to block EGFR, and 10 μm tyrphostin AG825 to block HER2 or the general Src kinase inhibitor PP2. C, HaCat human keratinocytes or MCF10A mammary epithelial cells are plated on 0.1 or 1 μg/ml LN332. D, HaCat or MCF10A cells are treated with EGF, or α6β4 integrin is clustered to activate the EGFR- or HER2-dependent integrin activation mechanisms. GoH3 or P1B5 is used to inhibit α6β4 or α3β1 binding to the laminin. E, MCF-10A cells are plated on 1 μg/ml LN332 under serum-free conditions, and the motility of individual cells is tracked over 10 h in the presence or absence of EGF. Relative migration distances are shown on spatial plot maps. Cells are treated with 10 μm AG825 to block HER2, 1 μm Iressa to block EGFR, or a combination of both inhibitors.