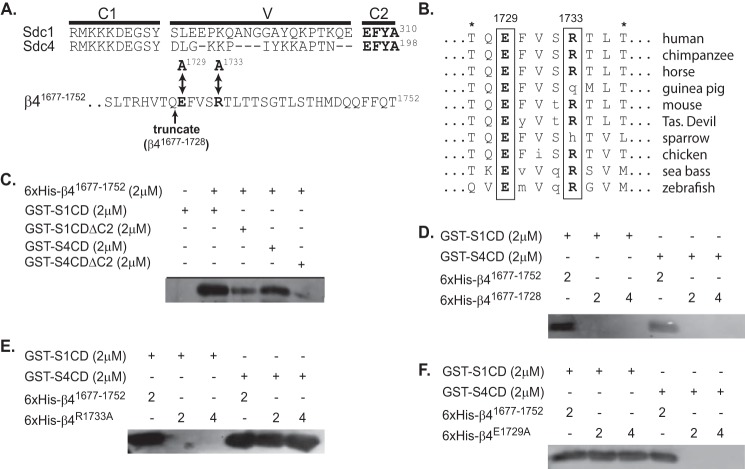
FIGURE 3.
Sdc1 and Sdc4 engage distinct sites at the C terminus of the β4 integrin cytoplasmic domain. A, complete cytoplasmic domains of human Sdc1 (S1CD) and Sdc4 (S4CD) are shown, denoting the juxtamembrane region conserved across the syndecan family (C1), the distal conserved region (C2) composed of the amino acids EFYA, and the V region unique to each syndecan family member. Also shown are the C-terminal 32 amino acids of the β4 integrin subunit contained in a His6-tagged β4 integrin construct (β41677–1752) used in binding studies. Double-headed arrows denote Glu-1729 and Arg-1733 (in bold), which were mutated to alanine to disrupt syndecan-specific binding to this construct. An arrow also denotes the site at which the construct is truncated to remove the last 24 amino acids (His6-β4(1677–1728)) necessary for syndecan binding. B, conservation of amino acids 1727–1736 in the β4 integrin cytoplasmic domain across species is shown, with conserved Glu-1729 and Arg-1733 in bold. Asterisks denote threonines that disrupt binding of the β4 cytoplasmic domain to plectin when phosphorylated (66). C, glutathione beads preincubated with 2 μm GST-S1CD, GST-S4CD, GST-S1CDΔC2, or GST-S4CDΔC2 were tested for their ability to capture 2 μm His6-β4(1677–1752), detected on Western blots with anti-penta-His. D–F, glutathione beads preloaded with GST-S1CD or S4CD are tested for the capture of either 2 or 4 μm His6-β4(1677–1752) or His6-β4(1677–1728) (D), or constructs in which point mutations have been introduced at Arg-1733 (His6-β4R1733A) (E) or Glu-1729 (His6-β4E1729A) (F).