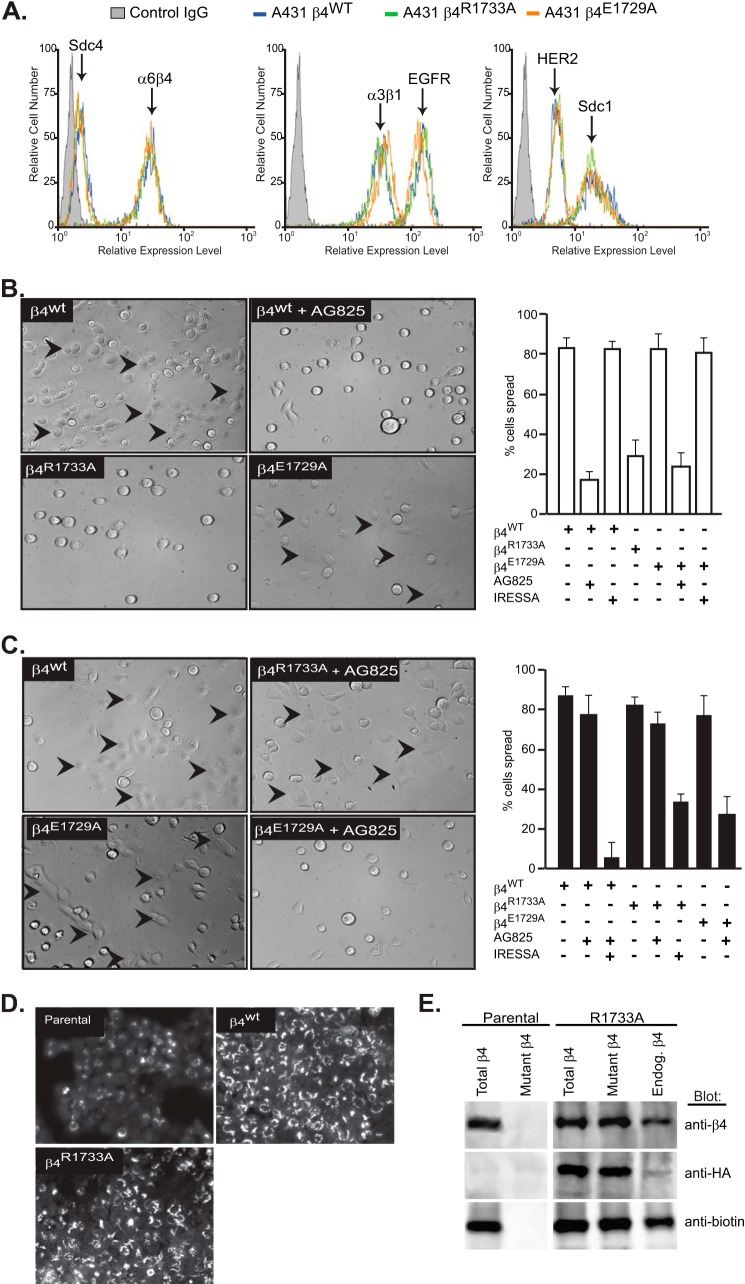
FIGURE 6.
α6β4R1733A and α6β4E1729A integrin constructs act as dominant negative mutants to block the HER2- and EGFR-dependent mechanisms, respectively, during A431 cell spreading. A, A431 cells expressing HA-tagged β4WT, β4R1733A, or β4E1729A constructs were subjected to flow cytometry to quantify relative expression of cell surface α6β4 integrin (3E1), Sdc1 (B-A38), and Sdc4 (F4–8G3), α3β1 integrin (ASC-1), and HER2 (9C6) or EGFR (EGFR.1). B, A431 cells expressing the β4WT, β4R1733A, or β4E1729A constructs are plated on 1 μg/ml LN332 for 2 h to activate the HER2-dependent signaling mechanism (spread cells denoted by arrowheads) with or without addition of 10 μm AG825 or 1 μm Iressa to inhibit HER2 or EGFR, respectively. The percentage of spread cells in triplicate wells from two experiments is quantified. C, A431 cells expressing β4 receptor constructs are plated on 1 μg/ml LN332 for 2 h in the presence of 10 ng/ml EGF with or without AG825 or Iressa and quantified as in B. D, A431 parental cells, β4WT-expressing cells, or β4R1733A-expressing cells are fixed, permeabilized, and stained for β4 integrin with mAb 3E1. E, relative amounts of endogenous α6β4 and HA-tagged α6β4R1733A (mutant) integrin at the cell surface is determined by biotinylation of parental or α6β4R1733A-expressing (R1733A) cells, followed by immunoprecipitation with mAb 3E1 to obtain “total β4,” with anti-HA (mAb 12CAS) to obtain “mutant β4,” or 3E1 precipitation of integrin remaining in the supernatant after anti-HA precipitation to quantify native endogenous β4. The relative amounts of total α6β4, HA-tagged α6β4R1733A, and biotinylated (cell surface) α6β4 integrin in each pool are assessed by staining a single blot with anti-β4 (AB1922), anti-HA (C29F4), and anti-biotin (212.26.A2) by stripping and reprobing.