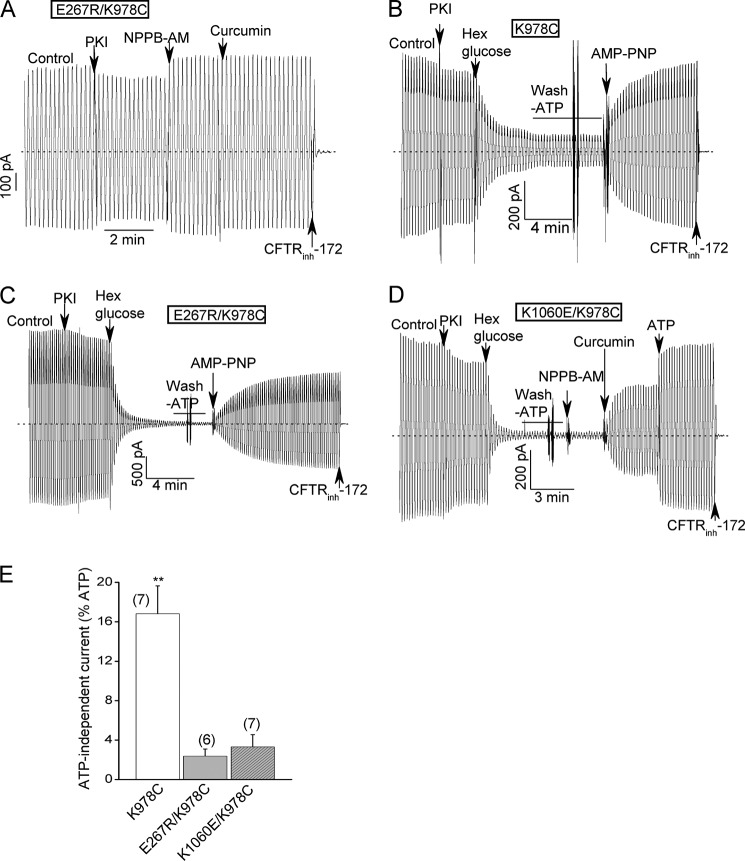
FIGURE 6.
Interplay between the charge- reversal mutations at the bundle interface and a previously reported GOF mutation (K978C); evidence that the E267R and K1060E mutations also inhibit ATP-free channel activity. A, E267R/K978C double mutant behaves more like wild type channels in the presence of ATP in macropatch experiments. Conditions were identical to Fig. 2A. The mean percent control currents of this double mutant when normalized to the maximal currents measured after potentiator addition were 94 ± 5.4% and 82.8 ± 3.9% before and after PKI addition, respectively (n = 5). These values are not smaller (in fact, somewhat larger) than those for WT-CFTR (see Fig. 2E.). B–D, macroscopic records for the indicated K978C single and double mutants showing that the E267R and K1060E substitutions decreased the fractional currents remaining after ATP removal. ATP was removed by the addition of an ATP scavenger (24 U/ml hexokinase plus 10 mm glucose) followed by bath perfusion with an ATP-free solution. E267R/K978C-CFTR was activated strongly by the subsequent addition of 2 mm AMP-PNP in the absence of ATP, as reported previously for the K978C GOF mutant (panels B, C and Ref. 47). E, mean percent ATP-free currents normalized to the currents before ATP removal for the indicated constructs. N is indicated in parentheses. **, p < 0.01 when compared with the double mutants by unpaired t test.