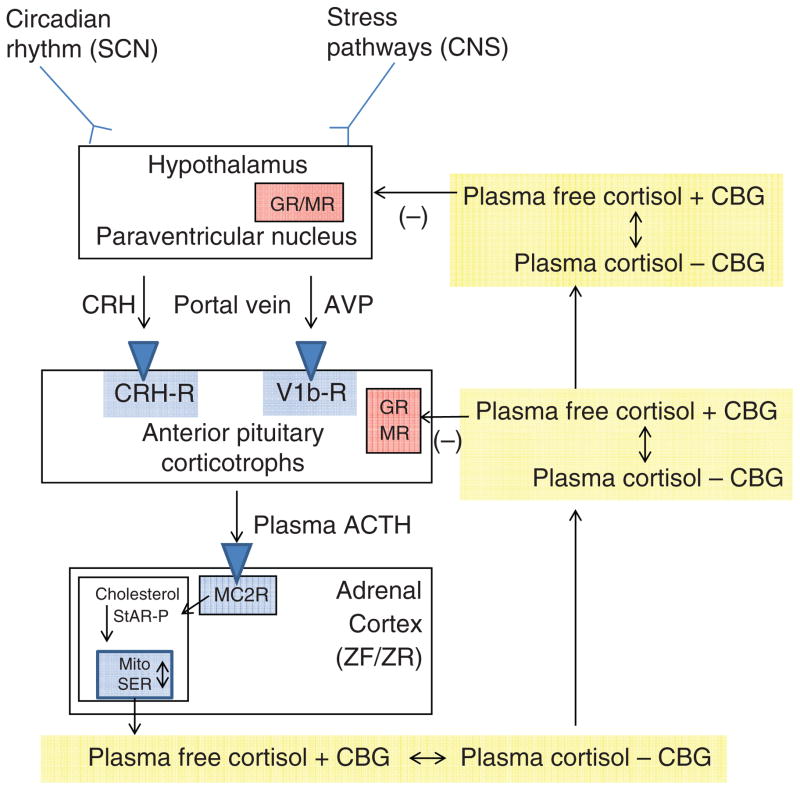
Figure 1.
The hypothalamic-pituitary-adrenal axis. Inputs from the hypothalamic circadian rhythm generator in the suprachiasmatic nucleus (SCN) and neural stress pathways in the central nervous system (CNS) control the activity of the corticotrophin-releasing hormone (CRH) neuronal cell bodies in the paraventricular nucleus. These neurons are also capable of synthesizing arginine vasopressin (AVP), which can augment the pituitary response to CRH. CRH (and AVP) are released into the portal circulation in capillaries in the median eminence and drain onto the anterior pituitary where they stimulate the pituitary corticotrophs to release adrenocorticotropic hormone (ACTH). ACTH stimulates the zona fasciculata (ZF) and zona reticularis (ZR) via the MC2R (melanocortin 2 receptor, also known as the ACTH receptor). This G-protein coupled receptor increases intracellular cAMP release, which activates StAR-mediated cholesterol transport into the mitochondria (the rate-limiting step of steroidogenesis). Once cholesterol reaches the inner mitochondrial (Mito) membrane, it is acted on by the first steroidogenic enzyme, and then by subsequent sequential enzymes in the smooth endoplasmic reticulum (SER) and Mito with cortisol as an end product (see Fig. 12). Cortisol is released into the plasma compartment where it binds reversibly to corticosteroid-binding globulin (CBG; also known as cortisol-binding globulin). As CBG-bound plasma cortisol enters the capillaries in target tissue, it dissociates from CBG and diffuses into the target cell. In the pituitary and hypothalamus, negative feedback inhibition is exerted with the binding of cortisol to glucocorticoid (GR) and mineralocorticoid (MR) receptors.