Abstract
Aims
To prospectively evaluate the reliability and efficacy of a new treatment for the reconstruction of the lacrimal duct using a new histo-engineered material, xenogeneic (bovine) acellular dermal matrix.
Method
Five patients (five eyes) with partial or total absence of the lacrimal duct were included in the study. Four patients (four eyes) suffered from traumatic injuries to the lacrimal duct and one patient (one eye) had congenital absence of the lacrimal drainage system. A pedal graft of conjunctiva was taken from the fornix area and rolled into a tube structure after being attached to the acellular dermal matrix.
Results
The average duration of follow-up for the patients was 7.2 months (ranging from 6 to 12 months). After surgery, the new duct in the nasal cavity could be observed above the middle turbinate by nasal endoscopy. Patency was confirmed by pressing in the area of the lacrimal sac and visualising air bubbles in the nasal cavity. Additionally, the meatus above the middle turbinate of the nasal cavity was stained and visualised after patients underwent Jones dye test 1 (JDT1). Five tear ducts proved to be effective through irrigation testing and epiphora symptoms were alleviated in all cases.
Conclusions
The newly reconstructed lacrimal duct, formed by the shift of autogenous conjunctival petal and the attachment of acellular dermal matrix, was successful in all five cases and suggests a new solution for the complex lacrimal duct lesion and congenital anomalies of the lacrimal duct.
Keywords: Lacrimal duct, Graft
Introduction
Lacrimal duct injury is a common problem in facial trauma, and it is often accompanied by angular deformity, eyelid injury or orbital fracture. The spectrum of severity ranges from simple lacrimal canalicular laceration to more complex injuries, such as distortion, laceration and displacement in the whole lacrimal drainage duct. Congenital absence of lacrimal puncta in children is a very rare condition. Congenital absence of bilateral whole lacrimal duct (the lack of the puncta, lacrimal canaliculi, lacrimal sac and nasolacrimal duct) is much rarer.1
The formation of a new duct for the treatment of these recalcitrant lacrimal system obstructions is especially challenging worldwide. Many previous studies took advantages of nasal mucoperiosteal flaps, venous vessels and oral mucosa etcetera to reconstruct new tear conduits. These studies chose Jones tube or extradural tube as the support in the new tube after surgeries.2–4 However, disadvantages relating to internal support were observed. In spite of the high rate of functional success in the early postoperative stage, these methods were noted to be associated with low rate of patient satisfaction and a number of complications. One such complication was prolonged inflammation resulting in ductal atresia and graft rejection necessitating removal. One study showed the use of Medpor glass tubes in rebuilding the lacrimal duct;5 however, this could cause chronic inflammation and tissue growth, leading to tube blockage and many patients in underdeveloped areas could not afford the great expenses of this tube. Therefore, exploring and creating an effective and reliable tear drainage system is an important area of development in the reconstruction of lacrimal drainage systems.6–9
The aim of this study is to explore a new treatment for the reconstruction of the lacrimal duct by using a histo-engineered material, xenogeneic (bovine) acellular dermal matrix, and prospectively evaluating the reliability and efficacy of this approach.
Materials and methods
Patients
A review was conducted for the recruitment of five patients (five eyes) with partial or total absence of the lacrimal duct. These patients were admitted to Sichuan Provincial People's Hospital during July 2012 and February 2013. Four patients suffered traumatic injuries to the lacrimal duct (four eyes) and one patient had a congenital absence of the lacrimal duct (one eye) (figure 1A–E). There were two male (two eyes) and two female (two eyes) patients with trauma (table 1). The fifth patient was a 9-year-old boy with a congenital abnormality of the tear duct. The four traumatic cases received operations 9 months to 1 year from when the accident occurred. All patients presented with complaints of epiphora.
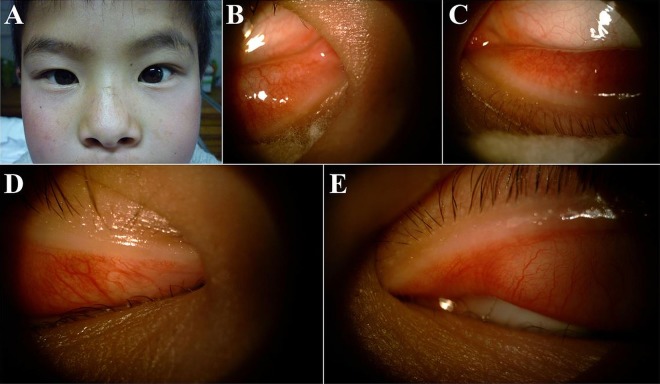
Figure 1.
A nine-year old boy with congenital absence of total lacrimal drainage. This patient has a normalexternal appearance (A) with absence of bilateral upper and lower lacrimal puncta (B–E).
Table 1 .
Clinical data of patients
| Patient | Gender | Age | Preoperation | Postoperation | ||
|---|---|---|---|---|---|---|
| Epiphora | Angular deformity | JDT1 test | ||||
| 1 | Female | 19 | Epiphora and angular deformity | Disappeared | Improved | (+) |
| 2 | Male | 24 | Epiphora and angular deformity and fracture | Improved | Improved | (+) |
| 3 | Female | 28 | Epiphora and angular deformity and fracture | Improved | Improved | (+) |
| 4 | Male | 33 | Epiphora | Disappeared | − | (+) |
| 5 | Male | 9 | Epiphora | Disappeared | − | (+) |
–, no angular deformity; +, positive result of the patients examined by JDT1 test; JDT, Jones dye test.
Methods
All cases underwent a complete eye examination including assessment for any associated ocular injuries. The four cases with traumatic lacrimal duct injury received probing of the lacrimal system; however, the probe could not completely pass through the upper and lower lacrimal canaliculus confirming lacrimal injury. A CT scan was used to show higher tissue density and bony pieces in the lacrimal sac area. Fracture and obstruction of the naso-lacrimal duct were found in these cases. The lacrimal puncta in the 9-year-old boy's eyes could not be found bilaterally on slit lamp examination (figure 1B–E). The colour Doppler ultrasonography examination for this patient showed no obvious lacrimal sac structure in both sides. Absence of lacrimal sac was suspected, and the right eye was chosen for operation at random.
Surgical materials included Heal-all Oral Biofilm, xenogeneic (bovine) acellular dermal matrix provided by Yantai Zhenghai Bio-Technology Co., Ltd (size: 2 cm×2.5 cm).
Procedures:
A skin incision was placed in the medial canthal region. A subcutaneous tunnel was created between the lacrimal fossa and the lacrimal caruncle (figure 2A).
A patch of 2.0 cm×0.8 cm conjunctival petal from the conjunctival fornix area that served as a free graft (figure 2B). The Heal-all Oral Biofilm was immersed in normal saline so that the conjunctival petal could attach to the oral biofilm (figure 2C).
The reconstructed complex of biofilm and conjunctival petal was rolled and fixed by suture into a tube-like structure, thus forming a duct with the conjunctival epithelium forming the inner wall and the biofilm the outer wall (figure 2D). The calibre of the complex conduit was approximately 2–3 mm length.
A bony window was created to expose the underlying nasal mucosa, which was cut in an ‘H’ shape.
The premade lacrimal duct was brought through the tunnel with one end fixed onto the lacrimal caruncle area, and the other end was anastomised with nasal mucosa. The centre area of the duct was fixed to the surrounding orbicular muscles by suture (figure 2E). Thus, a tear drainage conduit leading into the nose was successfully established.
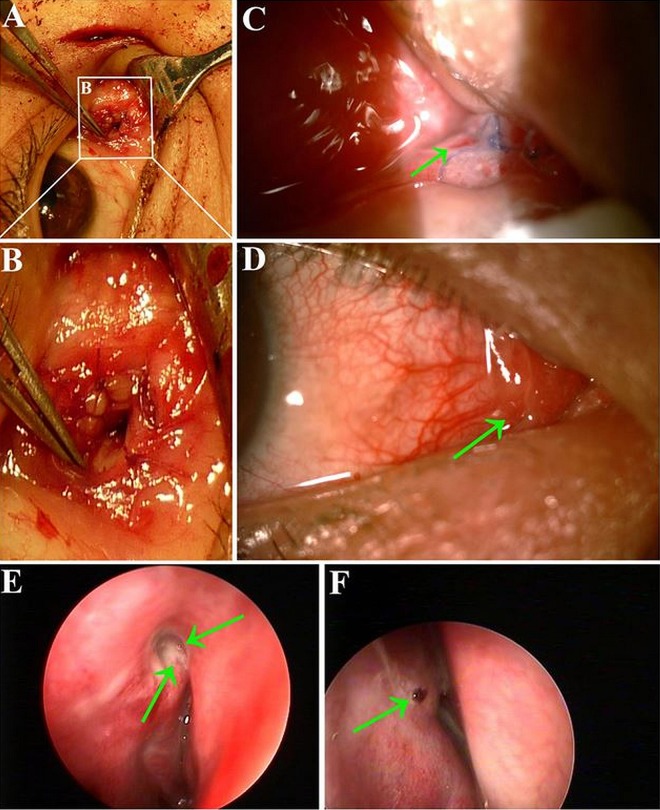
Figure 2.
The intraoperative pictures of the 9-year-old boy. There is a tunnel made from lacrimal caruncle through lacrimal sac area (A). The conjunctiva was harvested from inferior fornix (B). The conjunctiva was attached onto the oral biofilm (C). The oral biofilm was rolled into a tube shape with the conjunctiva being the internal lining (D). The newly-built duct was placed into the tunnel of lacrimal duct and fixed, with one end fixed onto the lacrimal caruncle and the other end fixed onto the nasal mucosa within the bony window (E).
Results
The average duration of follow-up for the five patients was 7.2 months (ranging from 6 to 12 months). Epiphora symptoms disappeared after surgery (table 1). The opening side of the newly reconstructed lacrimal duct could be seen in the lacrimal caruncle after surgery (figure 3A,B). It often appears half closed 1 week later (figure 3C) and is difficult to visualise under slit lamp exam in the subsequent period of observation (figure 3D). The nasal side of the conduit was visualised above the middle turbinate by nasal endoscopy. When the lacrimal sac area was pressed, air bubbles flowing out from the new duct were visualised on nasal endoscopy (figure 3E). For the Jones dye test 1 (JDT 1), an entire fluorescein 2% dose was dropped in eyes. After 5 min, the nasal mucosa was gently swabbed and fluorescein presence was confirmed (table 1), on nasal endoscopy the lacrimal duct meatus was noted to be stained with fluorescein, thus confirming its location (figure 3F). All patients felt bitter after 5 min when dropped 0.25% chloramphenicol eyedrops in the conjunctival sac.
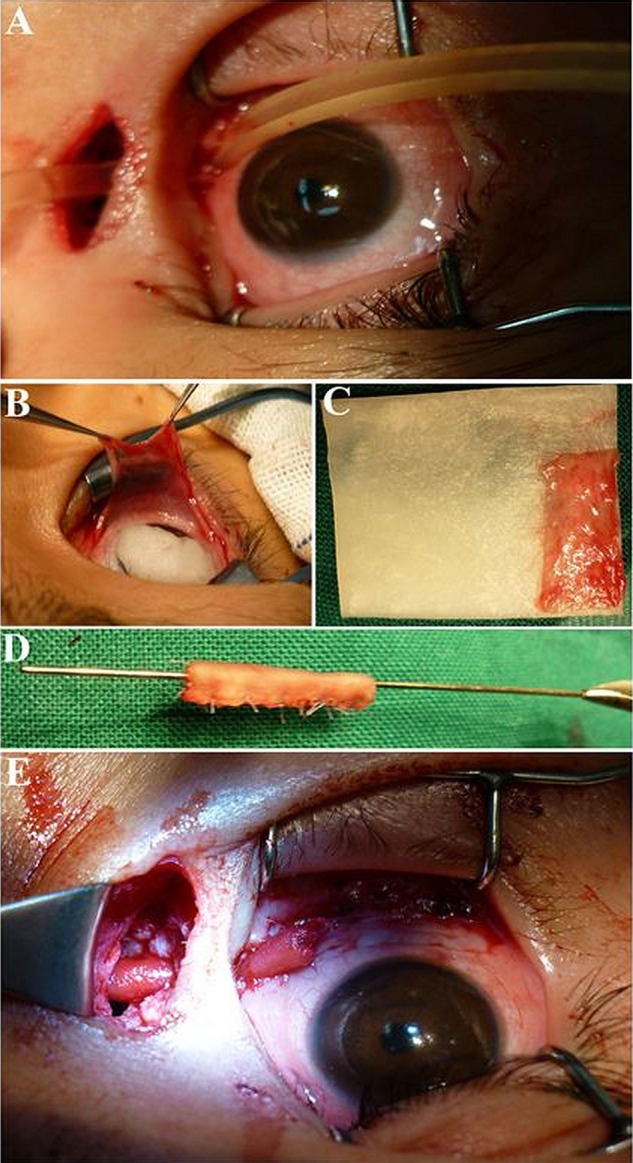
Figure 3.
Photograph of the opening end of the newly-built lacrimal ducts. The intra-operative picture shows the opening of newly-rebuilt lacrimal duct in the lacrimal caruncle (A). The amplification figure of the newly-rebuilt lacrimal duct in the lacrimal caruncle (B). The appearance of the tear conduit on the lacrimal caruncle 5 days after surgery. The arrow refers to the opening point with suture and swollen conjunctiva (C). The appearance of the lacrimal caruncle 1 year later after surgery. The arrow refers to the area, where it is difficult for us to find the opening point (D). The opening point of the new duct was observed above the middle turbinate under nasal endoscopy. When we pressed the lacrimal sac area, we could see the air bubbles flow out from the new duct vianasal endoscope (the green arrow, E). The arrow refers to the staining on the tear duct opening nasally visualized by nasal endoscopy after the JDT1 test (F).
Discussion
This study reports a new approach in the reconstruction of the lacrimal duct that addresses the issue of support for the new conduit as well as the histological structure of the inner wall on the lacrimal drainage systems. We used an outer support to enwrap the reconstructed lacrimal duct, xenogeneic (bovine) decellularised dermal matrix, which had a good cellular framework, certain elasticity and supportive features. Thus, the new conduit could maintain a stable position and patency for long-term tear drainage. We chose the conjunctival petal (2.0 cm×0.8 cm) as the inner wall of the new duct. This fistula tract has a large calibre and is lined entirely with normal mucosal epithelium. The patients were followed up for half a year, during which time the lacrimal duct was patent and epiphora symptoms had resolved.
Recently, major achievements in creating decellularised whole tissue scaffolds have drawn considerable attention as a promising approach for histology engineering. Decellularised tissues are expected to have mechanical strength and structure without antigen composition. Decellularised tissues have been reported to have the potential to regenerate a variety of tissues, such as skin, trachea, cardiac valve, heart, lung, liver, kidney, vascular and urethra.10–19 So far, there is no report about the application of decellularised dermal matrix in lacrimal duct reconstruction worldwide.
Surgical repair of the lacrimal drainage apparatus may be quite difficult in patients with maxillofacial injuries, resulting in extensive structural damage. When the primary tear tract become non-repairable or inaccessible, it is thus very necessary to set up an alternate draining route for tear passage.
As a bridge between the conjunctival sac and nasal cavity, the ideal artificial lacrimal duct drainage system should meet the following requirements: (1) the tear passage should maintain unobstructed; (2) no chronic proliferation of granulation tissue that can obstruct the duct or meatus; (3) the inner wall of lacrimal duct must have certain elastic tension and support to avoid adhesion and closure with the contraction of the orbicularis muscle; (4) the inner wall covered by mucosal tissue that has secretory and anti-infection qualities; (5) no immunogenicity and (6) it has abundant blood supply and can exist in the body over time without ocular irritation.
In the present study, we chose a new surgical method in lacrimal duct reconstruction. The inner wall of the lacrimal duct comes from autologous fornical conjunctiva that consists of simple columnar epithelium, thus making the newly built passage similar to the original tear duct in histology. Using conjunctiva is an effective method that has been confirmed in previous studies.20 21 However, the conjunctival mucosa flap is very thin and frail, making it in itself liable to external compression force, which may not always be satisfactory.
To support the newly built lacrimal duct, we used an artificial material, Heal-all Oral Biofilm (allograft acellular dermal matrix), a kind of dermal tissue with acellular disposal. This material provides a good cellular framework to preserve the natural structure of collagen, to repair the wound and reduce the scar formation. It possesses a certain elasticity and thickness; therefore, the conjunctival petal could be attached to the biofilm firmly. We rolled the two layers together into a tube shape and fixed it by suture, thus forming a conjunctival tube with outer support—a new lacrimal duct.
The oral biofilm is capable of degenerating and regenerating simultaneously. Thus, it will induce the pipe shape from the surrounding tissues in the first step, and when the mucosal duct reconstruction is finished it will degenerate automatically in the second step. After the autologous tissue takes shape, the scar proliferation and occlusion will not occur to the anastomosis of both ends of newly built lacrimal duct, and the newly formed lacrimal duct will not adhere and collapse.
In conclusion, this surgical method suggests a new solution for the complex lacrimal duct lesion and congenital anomalies of the lacrimal duct using acellular dermal matrix.
Acknowledgments
The authors thank all patients and family members for their participation.
Footnotes
Contributors: LC, ZW, CQ and RC: contact and interact with patients. BG: wrote the manuscript. JJ: corrected the manuscript.
Competing interests None
Funding: This study was supported by grants from the natural Science Foundation of China (81371048 to BG and 81100693 to CQ); the Department of Sichuan Provincial Health (130167 to BG).
Patient consent: Obtained.
Provenance and peer review: Not commissioned; externally peer reviewed.
References
- 1.Prabhakaran VC, Davis G, Wormald PJ, et al. Congenital absence of the nasolacrimal duct in velocardiofacial syndrome. J Aapos 2008;12:85–6. [DOI] [PubMed] [Google Scholar]
- 2.Athanasiov PA, Prabhakaran VC, Mannor G, et al. Transcanalicular approach to adult lacrimal duct obstruction: a review of instruments and methods. Ophthalmic Surg Lasers Imaging 2009;40:149–59. [DOI] [PubMed] [Google Scholar]
- 3.Lee JW. Nasolacrimal duct reconstruction with nasal mucoperiosteal flap. Ann Plast Surg 2007;59:143–8. [DOI] [PubMed] [Google Scholar]
- 4.Lim C, Martin P, Benger R, et al. Lacrimal canalicular bypass surgery with the Lester Jones tube. Am J Ophthalmol 2004;137:101–8. [DOI] [PubMed] [Google Scholar]
- 5.Fan XQ, Bi XP, Fu Y, et al. [The clinical study of lacrimal bypass surgery with high-density porous polyethylene coated tear drain implantation]. Zhonghua Yan Ke Za Zhi 2007;43:713–17. [PubMed] [Google Scholar]
- 6.Chaloupka K, Motwani M, Seifalian AM. Development of a new lacrimal drainage conduit using POSS nanocomposite. Biotechnol Appl Biochem 2011;58:363–70. [DOI] [PubMed] [Google Scholar]
- 7.Tao JP, Luppens D, McCord CD. Buccal mucous membrane graft-assisted lacrimal drainage surgery. Ophthal Plast Reconstr Surg 2010;26:39–41. [DOI] [PubMed] [Google Scholar]
- 8.Lin MT, Tsai CC, Lee SS, et al. A new method using epidural catheters in the reconstruction of lacrimal drainage. Scand J Plast Reconstr Surg Hand Surg 2005;39:85–9. [DOI] [PubMed] [Google Scholar]
- 9.Tsirbas A, Wormald P. Agger nasi cell mucosal autograft for lacrimal sac reconstruction during endonasal dacryocystorhinostomy. Orbit 2004;23:105–10. [DOI] [PubMed] [Google Scholar]
- 10.Whitfield RM, Rinard J, King D. Coverage of megaprosthesis with human acellular dermal matrix after Ewing's Sarcoma resection: a case report. Sarcoma 2011;2011:978617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Macchiarini P, Jungebluth P, Go T, et al. Clinical transplantation of a tissue-engineered airway. Lancet 2008;372:2023–30. [DOI] [PubMed] [Google Scholar]
- 12.Weymann A, Loganathan S, Takahashi H, et al. Development and evaluation of a perfusion decellularization porcine heart model--generation of 3-dimensional myocardial neoscaffolds. Circ J 2011;75:852–60. [DOI] [PubMed] [Google Scholar]
- 13.Filova E, Straka F, Mirejovsky T, et al. Tissue-engineered heart valves. Physiol Res 2009;58(Suppl 2):S141–58. [DOI] [PubMed] [Google Scholar]
- 14.Price AP, England KA, Matson AM, et al. Development of a decellularized lung bioreactor system for bioengineering the lung: the matrix reloaded. Tissue Eng Part A 2010;16:2581–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ozeki M, Narita Y, Kagami H, et al. Evaluation of decellularized esophagus as a scaffold for cultured esophageal epithelial cells. J Biomed Mater Res A 2006; 79:771–8. [DOI] [PubMed] [Google Scholar]
- 16.Shupe T, Williams M, Brown A, et al. Method for the decellularization of intact rat liver. Organogenesis 2010;6:134–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Nakayama KH, Batchelder CA, Lee CI, et al. Decellularized rhesus monkey kidney as a three-dimensional scaffold for renal tissue engineering. Tissue Eng Part A 2010;16:2207–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Williams C, Liao J, Joyce EM, et al. Altered structural and mechanical properties in decellularized rabbit carotid arteries. Acta Biomater 2009;5: 993–1005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Steger V, Hampel M, Trick I, et al. Clinical tracheal replacement: transplantation, bioprostheses and artificial grafts. Expert Rev Med Devices 2008;5:605–12. [DOI] [PubMed] [Google Scholar]
- 20.Struck HG, Ehrich D. Prognosis of conjunctivo-dacryocystorhinostomy. Modification with different implant materials. Ophthalmologe 2000;97:407–10. [DOI] [PubMed] [Google Scholar]
- 21.Kominek P, Cervenka S, Matousek P. Bilateral simultaneous dacryocystorhinostomy and conjunctivocystorhinostomy. Cesk Slov Oftalmol 2008;64:91–4. [PubMed] [Google Scholar]