Abstract
Background
The lung volume reduction (LVR) coil is a minimally invasive bronchoscopic nitinol device designed to reduce hyperinflation and improve elastic recoil in severe emphysema. We investigated the feasibility, safety and efficacy of LVR coil treatment in a prospective multicentre cohort trial in patients with severe emphysema.
Methods
Patients were treated in 11 centres. Safety was evaluated by recording all adverse events, efficacy by the St George's Respiratory Questionnaire (SGRQ) as primary endpoint, and pulmonary function testing, modified Medical Research Council dyspnoea score (mMRC) and 6-min walk distance (6MWD) up to 12 months after the final treatment.
Results
Sixty patients (60.9 ± 7.5 years, forced expiratory volume in 1 s (FEV1) 30.2 ± 6.3% pred) were bronchoscopically treated with coils (55 bilateral, 5 unilateral), with a median of 10 (range 5–15) coils per lobe. Within 30 days post-treatment, seven chronic obstructive pulmonary disease exacerbations (6.1%), six pneumonias (5.2%), four pneumothoraces (3.5%) and one haemoptysis (0.9%) occurred as serious adverse events. At 6 and 12 months, respectively, ΔSGRQ was −12.1±12.9 and −11.1±13.3 points, Δ6MWD was +29.7±74.1 m and +51.4±76 m, ΔFEV1 was +0.11±0.20 L and +0.11±0.30 L, and ΔRV (residual volume) was −0.65±0.90 L and −0.71±0.81 L (all p<0.01). Post hoc analyses showed significant responses for SGRQ, 6MWD and RV in patients with both heterogeneous and homogeneous emphysema.
Conclusions
LVR coil treatment results in significant clinical improvements in patients with severe emphysema, with a good safety profile and sustained results for up to 1 year.
Trial registration number:
Keywords: Emphysema, Bronchoscopy
Key messages.
What is the key question?
Is lung volume reduction coil treatment feasible and does it sustainably improve quality of life and clinical outcomes in a broad group of patients with severe emphysema treated in a multicentre setting?
What is the bottom line?
Bronchoscopic lung volume reduction coil treatment is associated with a good safety profile and significantly improves quality of life, exercise capacity and pulmonary function in a broad group of patients with severe emphysema, with sustained results at 1 year.
Why read on?
Further post hoc analysis of CT scan heterogeneity showed significant responses in both heterogeneous and homogeneous emphysema, suggesting that lung volume reduction coil treatment may benefit patients with both heterogeneous and homogeneous emphysema disease distribution.
Introduction
For patients with advanced chronic obstructive pulmonary disease (COPD) who, despite optimal medical management still have severe dyspnoea, bronchoscopic lung volume reduction could be a beneficial treatment option.1 2 Although lung volume reduction surgery and lung transplantation are still valid treatment modalities for patients with COPD, the use of these interventions is very limited because of strict patient selection criteria, significant morbidity and donor shortage.3–5
To date, bronchoscopic lung volume reduction using one-way endobronchial valves (EBV) has been the most extensively investigated technique in this field.6–8 However, successful clinical outcomes from EBV therapy can only be achieved in patients with no interlobar collateral ventilation and when the one-way valves are placed to entirely block all the airways into the target lobe, which can be technically difficult due to local anatomy and in the absence of significant experience with these devices.6–8 It is estimated that only about 33% of patients with severe emphysema have no collateral ventilation between the target and adjacent lobe and can thus potentially be treated using one-way valves.1 This clearly shows the need for alternative bronchoscopic treatments that work independently of the presence of collateral ventilation.
In 2010 we reported the first human trial using bronchoscopically delivered nitinol lung volume reduction (LVR) coils.9 Up to six shape-memory coils per lung were placed in patients with severe emphysema, resulting in moderate effects only in the patients with heterogeneous emphysema but without any serious adverse events. After that first trial we improved the LVR coil treatment to target the most diseased areas of the lung with approximately 10 coils placed per lobe, in order to maximise re-tensioning of the airway network. The results using this approach in 16 patients with upper lobe predominant heterogeneous emphysema have previously been published, showing feasibility and safety and also demonstrating statistically and clinically significant improvements in pulmonary function, exercise capacity and quality of life.10 Surprisingly, even in this early pilot phase, two-thirds of the patients treated responded beyond the minimal clinically important differences (MCID) for forced expiratory volume in 1 s (FEV1),11 residual volume (RV),12 6-min walk distance (6MWD)13 and St George's Respiratory Questionnaire (SGRQ).14
Following the successful early experiences in these two pilot trials, the current study allowed further investigation into the feasibility, safety and efficacy of LVR coil treatment in a multicentre setting in a larger group of patients.
Methods
This prospective open-label multicentre feasibility study was conducted in 11 hospitals in France, Germany and the Netherlands and was approved by the ethics committee at each site. The first patient was enrolled in December 2009 and the final patient in October 2011. The initial protocol proposed a follow-up period of 6 months following initial treatment. However, because the Dutch and French ethics committees required a 12-month follow-up period, the protocol was modified to require a 12-month follow-up for patients in the Netherlands and France, while maintaining the original 6-month follow-up period for patients in Germany. This paper reports on all patients in the study at both exit points.
Patients
Patients with COPD with upper or lower lobe predominant bilateral heterogeneous emphysema on chest CT scan as judged by the treating physician were considered for inclusion. All patients were intended to be treated bilaterally, in accordance with the protocol assessment schedule. The inclusion and exclusion criteria are presented in box 1.
Box 1 Main study inclusion and exclusion criteria.
Main inclusion criteria
>35 years of age
CT scan indicates bilateral heterogeneous emphysema
Post-bronchodilator FEV1 <45% of predicted
Total lung capacity >100% of predicted
RV >175% of predicted
mMRC >2 (0–4)
Stopped smoking for >8 weeks prior to entering the study
Main exclusion criteria
Change in FEV1 >20% post-bronchodilator
Tlco <20% of predicted
History of recurrent clinically significant respiratory infection
Pulmonary hypertension: right ventricular pressure >50 mm Hg
Inability to walk >140 m in 6 min
Previous LVR surgery, lung transplant or lobectomy
Clinically significant bronchiectasis
Giant bullae more than one-third lung volume
Severe destructed homogeneous emphysema by CT scan
Patient on antiplatelet agent (eg, clopidogrel) or anticoagulant therapy (eg, heparin or coumadin) or has not been weaned off prior to procedure
FEV1, forced expiratory volume in 1 s; LVR, lung volume reduction; mMRC, modified Medical Research Council dyspnoea score; RV, residual volume; Tlco, carbon monoxide lung transfer factor.
Lung volume reduction (LVR) coil treatment
LVR coil treatment was performed as previously described.10 Briefly, the RePneu LVR coil (PneumRx, USA) (figure 1) is an implantable device composed of preformed nitinol wire which is straightened for delivery via a therapeutic flexible bronchoscope into subsegmental airways using a special delivery catheter, cartridge and loading forceps. Once in place, it is released and recovers to a non-straight predetermined shape upon deployment. Seven sizes of coil were available (70, 85, 100, 125, 150, 175 and 200 mm). All procedures were performed under general anaesthesia and the deployment of the coil was visualised under fluoroscopy. The coils were deployed with the objective of achieving equal subsegmental distribution throughout one target lobe. The contralateral procedure was performed at least 1 month after the first procedure.
Figure 1.
Fully deployed nitinol RePneu lung volume reduction coil.
Assessments and follow-up
Screening assessments included medical history, physical examination, dyspnoea assessment by the modified Medical Research Council dyspnoea scale (mMRC), quality of life assessment by the SGRQ,15 echocardiogram, pre- and post-bronchodilator spirometry, lung volume measurements by body plethysmography,16 6MWT,17 chest X-ray and a thoracic CT scan.
The patient was kept at least overnight after the procedure. A 1-month follow-up evaluation was performed, after which the second procedure was scheduled. Patients were then followed at 1, 3, 6 and 12 months (the latter only in France and The Netherlands).
Primary/secondary endpoints and safety objectives
The primary efficacy endpoint was the improvement in SGRQ total score from baseline compared with the score at 6 months. The secondary efficacy endpoints were the comparison between baseline and 6 months for forced vital capacity (FVC), FEV1, RV, residual volume to total lung capacity (RV/TLC) ratio, improvement in 6MWD and mMRC score. The responder rate at 6 months was calculated using the MCID defined for FEV1,11 RV,12 6MWD13 and SGRQ.14
The safety objectives were to identify the number and type of device-related and procedure-related adverse events related to the use of the LVR coil.
Post hoc CT scan analyses
Since inclusion in this trial was based on the treating physicians’ visual chest CT judgement, a post hoc analysis was performed on these CT scans to analyse the relationships between the response to LVR coil treatment at 12 months follow-up and the level of heterogeneity assessed by a blinded qualitative visual 4-point tissue destruction score scale (0–25%, 26–50%, 51–75%, >75% visible tissue destruction), as well as by calculating the percentage area of destruction below −950 Hounsfield Units (HU) between the upper and lower lobes of both lungs. Quantitative CT analyses were blinded and performed with CIRRUS Lung 13.10 (Diagnostic Image Analysis Group Nijmegen, The Netherlands; Fraunhofer MEVIS, Bremen, Germany). The lungs and lobes were automatically segmented and visually inspected. Emphysema was quantified per lobe as an emphysema score: the percentage of voxels below −950 HU.18
For the visual assessment, a patient was classified as heterogeneous if there was a difference of >1 point between ipsilateral lobes on both sides. For the computerised assessment, a patient was classified as heterogeneous when the difference for both lungs in the lung tissue destruction score was >25% at −950 HU between ipsilateral upper and lower lobes.
Statistics
This trial was powered on the statistical significant difference in expected SGRQ total score between baseline and the 6-month follow-up time point using an α<0.05 with a power of 0.90, taking a patient loss to follow-up of 10% into account.10
Data are presented as mean±SD, except for the presentation of the five unilateral cases and descriptive statistics on the detailed procedural results (table 2) where data are expressed as median (minimum−maximum) or median when appropriate. The statistical significance of changes from baseline was assessed by the paired Student t test. A linear regression analysis was performed to associate outcome at 6 months for changes in SGRQ and 6MWD, using as baseline regressors RV% pred, RV/TLC, FEV1% pred, FVC, age, carbon monoxide lung transfer factor (Tlco) and emphysema type (homogeneous or heterogeneous disease). The models were simple linear with no interactions or terms higher than first order included; p<0.05 was considered statistically significant. SAS V.9.3 was used for all analyses. All data in this trial were independently monitored by a contract research organisation.
Table 2.
Lung volume reduction coil procedural results
| Number of procedures | 115 |
| Procedure time, min | |
| Mean | 49.9±23.2 |
| Median | 45.0 (20–135) |
| Post-procedure hospital stay, days | |
| Mean | 2.3±2.8 |
| Median | 1.0 (0–19) |
| Coils per procedure, n | |
| Mean | 9.8±1.4 |
| Median | 10 (5–15) |
| Total coils implanted | 1125 |
| Upper, right lobe | 437 |
| Upper, left lobe | 450 |
| Lower, right lobe | 110 |
| Lower, left lobe | 121 |
| Middle, right lobe | 7 |
| LVR coil implant size | |
| 70 mm | 5 |
| 85 mm | 20 |
| 100 mm | 508 |
| 125 mm | 462 |
| 150 mm | 101 |
| 175 mm | 28 |
| 200 mm | 1 |
Dataare shown as numbers, mean±SD or median (minimum − maximum).
LVR, lung volume reduction.
Results
Patients and procedures
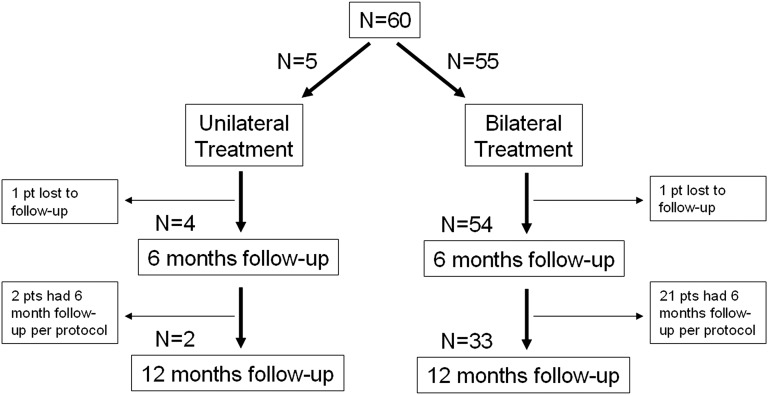
Sixty patients were enrolled between December 2009 and October 2011 and their baseline demographics are shown in table 1). A total of 115 procedures were performed (5 patients had unilateral treatment, figure 2) in which a total of 1125 LVR coils were placed. A median of 10 coils (range 5–15) was placed per lobe (table 2).
Table 1.
Patient demographics and baseline characteristics (n=60)
| Gender F/M | 33/27 |
| Age, years | 60.9±7.5 |
| Pack-years | 39.5±18.2 |
| BMI, kg/m2 | 24.92±4.49 |
| FEV1, L | 0.83±0.25 |
| FEV1, % pred | 30.17±6.32 |
| FVC, L | 2.49±0.78 |
| FVC, % pred | 73.95±16.94 |
| FEV1/FVC | 0.34±0.07 |
| RV, L | 5.29±1.32 |
| RV, % pred | 249.2±53.2 |
| RV/TLC | 65.55±8.19 |
| 6MWD, m | 316±102 |
| SGRQ, points | 61.5±14.3 |
| Supplemental oxygen | 35/60 (58%) |
| mMRC, points | 3.0±0.75 |
Data are shown as mean±SD.
BMI, body mass index; FEV1, forced expiratory volume in 1 s; FVC, forced vital capacity; mMRC, modified Medical Research Council dyspnoea score; 6MWD, 6-min walking distance; RV, residual volume; RV/TLC, residual volume to total lung capacity ratio; SGRQ, St George's Respiratory Questionnaire total score.
Figure 2.
Study flow chart.
Safety
No periprocedural serious adverse events occurred in the 115 bronchoscopies performed under general anaesthesia. No death or respiratory failure was reported. A summary of all serious and non-serious respiratory adverse events is listed in table 3. All events were treated and resolved with routine medical care and without sequelae.
Table 3.
Adverse events
| Treatment–1 month | >1–6 months | >6–12 months | ||||
|---|---|---|---|---|---|---|
| Events | Patients | Events | Patients | Events | Patients | |
| Serious respiratory adverse events | ||||||
| COPD exacerbation | 7 | 7 | 12 | 10 | 4 | 3 |
| Pneumonia | 6 | 5 | 3 | 3 | 6 | 6 |
| Haemoptysis | 1 | 1 | 0 | 0 | 0 | 0 |
| Pneumothorax | 4 | 4 | 2 | 2 | 1 | 1 |
| Respiratory adverse events | ||||||
| COPD exacerbation | 8 | 7 | 21 | 15 | 19 | 15 |
| Pneumonia | 5 | 3 | 4 | 3 | 3 | 3 |
| Mild haemoptysis (<5 mL) | 61 | 35 | 3 | 3 | 2 | 2 |
| Cough | 2 | 2 | 3 | 3 | 0 | 0 |
| Transient chest pain | 28 | 20 | 7 | 6 | 3 | 3 |
Adverse events presented per procedure for the first month after each procedure (115 procedures in total), for patients in the 1–6 month follow-up period (n=58) and for patients in the 6–12 month follow-up period (n=35). Events reported for both unilateral and bilateral treated patients.
COPD, chronic obstructive pulmonary disease.
Efficacy
All patients
Of the 60 patients who were bilaterally treated, 58 were evaluable at 6 months and 34 at 12 months (24 patients from Germany exited the study at 6 months). Because the German cohort exited the study at 6 months, we segregated the data to compare patients with 1-year follow-up data against their own 6-month results to analyse the sustainability of the clinical improvements within the same population (table 4). Across key clinical parameters, FEV1% pred, RV% pred and SGRQ results were sustained while mean 6MWD actually improved between 6 and 12 months. The MCID responder percentages for FEV1, RV, 6MWD and SGRQ are shown in table 5.
Table 4.
Efficacy results at 6 and 12 months
| 6 Months | 6 Months | 12 Months | |
|---|---|---|---|
| Overall group (N=58) |
12-month follow-up group (N=34) |
12-month follow-up group (N=34) |
|
| FEV1, L | +0.11±0.20 (n=54, p<0.001) | +0.12±0.28 (n=33, p=0.021) | +0.11±0.30 (n=34, p=0.037) |
| FEV1, % pred (% change) | +15.36±26.68 (n=54, p<0.001) | +17.81±31.71 (n=33, p=0.003) | +16.04±35.54 (n=34, p=0.017) |
| FVC, L | +0.20±0.53 (n=54, p=0.008) | +0.33±0.57 (n=33, p=0.002) | +0.28±0.45 (n=34, p=0.001) |
| RV, L | −0.65±0.90 (n=58, p<0.001) | −0.80±1.03 (n=34, p<0.001) | −0.71±0.81 (n=34, p<0.001) |
| RV, % pred (% change) | −11.31±15.25 (n=58, p<0.001) | −14.38±15.42 (n=34, p<0.001) | −13.75±12.65 (n=34, p<0.001) |
| RV/TLC | −4.51±12.19 (n=58, p=0.007) | −6.06±8.58 (n=34, p<0.001) | −3.12±15.59 (n=34, p=0.245) |
| 6MWD, m | +29.7±74.1 (n=56, p=0.004) | +42.4±73.5 (n=34, p=0.002) | +51.4±76.1 (n=32, p=0.003) |
| SGRQ, points | −12.1±12.9 (n=56, p<0.001) | −10.4±15.8 (n=33, p<0.001) | −11.1±13.3 (n=32, p<0.001) |
| mMRC, points | −0.6±1.2 (n=58, p<0.001) | 0.8±0.9 (n=34, p<0.001) | −0.7±0.8 (n=34, p<0.001) |
Efficacy at 6 months for all LVR coil treatments (n=58, overall group) and at 6 and 12 months (n=34, 12-month follow-up group columns). Results are given as change from baseline. Data are shown as mean±SD.
Data in parentheses are the numbers of actual measurements available per variable tested followed by the actual p value.
6MWD, 6-min walking distance; FEV1, forced expiratory volume in 1 s; FVC, forced vital capacity; mMRC, modified Medical Research Council dyspnoea score; RV, residual volume; SGRQ, St George's Respiratory Questionnaire total score; TLC, total lung capacity.
Table 5.
Responder rates at 6 and 12 months
| Variable | MCID | 6 months (%) | 12 months (%) |
|---|---|---|---|
| FEV1 | ≥12%11 | 48.0 | 40.6 |
| RV | ≥0.3512 | 64.8 | 57.6 |
| 6MWD | ≥26 m13 | 52.8 | 60.0 |
| SGRQ | ≥4 points14 | 74.1 | 65.6 |
| SGRQ | ≥8 points | 61.1 | 53.1 |
Responder rates at 6 and 12 months after bilateral lung volume reduction coil treatment using minimal clinically important differences (MCID) for forced expiratory volume in 1 s (FEV1), residual volume (RV), 6-min walking distance (6MWD) and the St George's Respiratory Questionnaire total score (SGRQ). Results are given as percentage of responders to total patients.
Unilateral patients
Five patients were treated unilaterally. The reasons for treating only one lung were: lost to follow-up in two patients; second lung on second look not suitable for treatment (bullae) in one patient; and second lung declined by two patients (one improved satisfactory and one did not want to proceed with the trial). At 6-month follow-up in four evaluable patients, the median change in FEV1 was +4.7% (range −17.8% to +17.0%), median change in 6MWD was +29 m (range −46 m to +92 m) and RV and RV/TLC remained stable.
Heterogeneous versus homogeneous disease
In the 33 bilaterally treated patients with 12 months follow-up, the post hoc visual qualitative CT score of the degree of tissue destruction classified 20 patients as heterogeneous and 13 as homogeneous. When using the CT software analysis, 16 patients were classified as heterogeneous and 17 as homogeneous. Regardless of the classification method, both heterogeneous and homogeneous patients showed significant improvement at 1 year (table 6).
Table 6.
Results at 12 months after bilateral LVR coil treatment for patients classified as heterogeneous and homogeneous emphysema
| Visual CT assessment* | Digital CT assessment* | |||||
|---|---|---|---|---|---|---|
| (12 month follow-up group) | (12 month follow-up group) | |||||
| Heterogeneous (n=20) | Homogeneous (n=13) | p Value | Heterogeneous (n=16) | Homogeneous (n=17) | p Value | |
| ΔFEV1, L | +0.14±0.30 | +0.08±0.28 | 0.585 | +0.18±0.32 | +0.05±0.26 | 0.220 |
| ΔRV, L | −0.69±0.87 | −0.68±0.46 | 0.859 | −0.75±0.78 | −0.66±0.72 | 0.719 |
| Δ6MWD, m | +53.9±65.1 | +46.0±67.9 | 0.739 | +74.9±67.4 | +27.9±57.8 | 0.049 |
| ΔSGRQ, points | −12.9±15.1 | −7.3±8.7 | 0.187 | −12.4±13.9 | −9.1±12.9 | 0.491 |
Results are given as mean±SD change from baseline. Heterogeneity and homogeneity were assessed by both a visual CT assessment (a 4-point qualitative score of the degree of tissue destruction where a difference of ≤1 point for both lungs was regarded as homogeneous) and a digital CT assessment (where the software calculated the percentage area of destruction at –950 Hounsfield Units; a difference of ≤25% in destruction for both lungs was regarded as homogeneous).
6MWD, 6-min walking distance; FEV1, forced expiratory volume in 1 s; LVR, lung volume reduction; RV, residual volume; SGRQ, St George's Respiratory Questionnaire total score.
*p<0.05 for all end-points compared to baseline.
Upper versus lower lobe disease
In this trial lower lobe treatment was performed in 10 patients, of whom nine could be evaluated at the 6-month endpoint. Except for FEV1 (+0.04±0.08 L for lower lobe vs +0.15±0.23 L for upper lobe; p=0.026), there were no statistically significant differences in the clinical responses between patients with upper versus lower lobe disease for RV, 6MWD and SGRQ.
Responder analysis
To identify LVR coil treatment responders we performed a multivariable analysis for the primary endpoint SGRQ and for the 6MWD. None of the input regressors (RV% pred, RV/TLC, FEV1% pred, FVC, age, Tlco and emphysema type) were useful in associating patient outcomes at 6 months.
Discussion
This prospective multicentre study assessed the long-term safety and improvements in patient-related outcome measures of LVR coil treatment in 60 patients with severe emphysema. The results show an acceptable safety profile associated with a significant and sustained improvement over 12 months in relevant clinical and functional parameters including FEV1, RV, 6MWD and SGRQ.
This is the largest LVR coil study to date, and also evaluated longer-term results of LVR coil treatment. In our first pilot study (n=16) using the current treatment approach (median 10 coils per lung) and coil design, significant clinical and functional improvements were seen at 6 months including SGRQ (−14.9 points), FEV1 (+14.9%), RV (−11.4%) and 6MWD (+84 m) with an acceptable safety profile.10 Recently, Shah et al19 reported the results at 90 days after bilateral LVR coil treatment for 46 patients included in a randomised controlled study and demonstrated a significant improvement in SGRQ (−8.1 points), FEV1 (+14%), RV (−0.51 L) and 6MWD (+51 m), with no difference in serious adverse events between treatment and control groups.
In the present multicentre study involving 11 centres, no serious adverse events were reported during the LVR coil treatment procedures, demonstrating procedural safety. Serious adverse events (table 3) mainly occurred in the 30 days after the procedure, with all events resolving with regular medical care and without sequelae. Our results confirm the acceptable safety profile for LVR coil treatment with a rate of adverse events similar to previous reports on LVR coil treatment.10 19 The rate of post-procedure exacerbations and pneumonia is comparable to reported events with endobronchial one-way valves.6 8 Importantly, the total rate of these COPD-related events following endoscopic implants did not exceed the number of exacerbations and pneumonia that were reported in the EASE trial sham bronchoscopy control group.20 LVR coil specific procedure-induced events that occur are typically very mild haemoptysis or coloured sputum requiring no intervention in about 50% of subjects and temporary chest discomfort for a few days requiring either a standard painkiller regimen for a few days or no intervention at all in about one-third of subjects treated.
Regarding efficacy, our results show significant improvements in clinical and functional parameters at 6 months with a magnitude of response in line with the two recent reports on LVR coil treatment,10 19 reporting on 6- and 3-month follow-up, respectively. Our study provides the first longer-term analysis of data over 12 months after bilateral LVR coil treatment and demonstrates a sustained response at 12 months. To better analyse the relevance of the efficacy results, we analysed the MCID in FEV1,11 RV,12 6MWD13 and SGRQ14 and found a significant responder rate at 6 and 12 months for these clinical endpoints (table 5).
The cohort trial design can, of course, induce bias. However, the results reported are higher than reported MCID for our endpoints and show similar efficacy across multiple centres. Furthermore, we have previously shown that, even in a sham-controlled bronchoscopic interventional trial design, no real placebo effect could be observed in patients with severe COPD.20
To better understand the predictors of response to LVR coil treatment, we conducted a multivariate analysis to assess the relationship between the response to treatment and baseline variables typically identified as predictors of outcome, such as hyperinflation and emphysema heterogeneity. Using the 6-month endpoints, none of the evaluated baseline variables provided a meaningful predictor of response to LVR coil treatment. Other potential variables could include nuanced emphysema phenotypes beyond heterogeneous or homogeneous classification, such as more or less small airways disease, centrilobular versus panlobular emphysema and variability in placement strategies including proximal versus distal placement within the subsegmental airways and/or the number and size of coils deployed. The current active clinical trials (NCT0182279521 and NCT01608490) and future meta-analysis data of patients treated in the four European clinical studies thus far may increase the statistical power sufficiently to perform this analysis better. In our study, where broad selection criteria were purposely used in order to evaluate the effectiveness in a population of patients representative of the patients we see in daily practice, we found a large variability of response between patients. However, responder rates overall for several endpoints were already high (table 5).
The difficulty of identifying strong predictors of success has been previously demonstrated by a predictive multivariate effort completed in a much larger patient cohort (n=608) for outcome after lung volume reduction surgery. In this large group, only a very weak signal for RV/TLC and emphysema distribution could be demonstrated.22
Lung hyperinflation is a major feature of emphysema and is associated with dyspnoea, exercise intolerance and compromised daily physical activity.23 24 In this study, neither baseline RV nor RV/TLC predicted the response to LVR coil treatment. This is possibly due to the fact that RV >175% pred was an inclusion criterion, resulting in treatment of severe static hyperinflated patients (mean baseline RV 249.2±53.2% pred). On the other hand, the magnitude of change in RV after LVR coil treatment was associated with more favourable mean clinical and functional outcomes in this study, suggesting that RV changes may be viewed as a marker of response to treatment and that, by selecting patients with more potential for significant RV decrease, the likelihood of significant clinical benefit may be increased. The finding that RV is reduced by LVR coil treatment might be related to mechanical volume compression of lung tissue exerted by the coils, as well as improvement in elastic recoil achieved by decreasing airway resistance.25
When comparing the results for patients with upper lobe versus lower lobe treatment, no outcome differences were observed for RV, 6MWD and SGRQ. The lower FEV1 results seen with lower lobe coil treatments is comparable to the experience with lung volume reduction surgery in the lower lobes where the effect on improving FEV1 is also limited compared with other outcome variables.26 However, because FEV1 in general shows poor correlation with performance in patients with severe emphysema,27 and that patient-relevant outcomes such as 6MWD and SGRQ show strong improvement even in lower lobe subjects, lower lobe treatment with coils appears to be a clinically valid treatment option with clear patient benefit. Future work will evaluate whether, as currently hypothesised, the much bigger lower lobes require a greater number of coils to optimise results.
Our post hoc CT analysis showed that a large number of patients were classified as homogeneous when using both a visual and a digital assessment, even though the inclusion criteria called for heterogeneous patients per clinicians’ visual assessment. This finding should be cautiously considered, since this trial was not designed to prospectively identify homogeneous emphysema patients and the two methods of creating a heterogeneous versus a homogeneous group are arbitrary. Our results show that LVR coil treatment also benefits patients with less pronounced heterogeneous to homogeneous disease. Our data showed a statistically and clinically significant benefit for both groups compared with baseline, with overall a potentially increased mean efficacy for the heterogeneous patient group. The fact that LVR coil treatment also shows efficacy in patients with homogeneous emphysema is a very important finding, challenging the assumption that only patients with heterogeneous emphysema will respond to LVR coil treatment, as has been shown for surgical lung volume reduction28 and EBV.6 7 Of note, other bronchoscopic techniques such as thermal vapour ablation29 and sealant therapy30 are also restricted to upper lobe predominant heterogeneous emphysema, leaving a broad group of patients with non-upper lobe predominant and homogeneous disease without a treatment option. It can be hypothesised that LVR coil treatment is similarly efficient in both heterogeneous and homogeneous emphysema because of a different mechanism of action from true ‘lung volume reducing’ therapies, as the primary mechanism of action of coils appears to be mechanical re-tensioning of the airway network rather than just reducing absolute lung volume alone. However, additional studies are necessary to better characterise the mechanisms of action of coils and also to confirm the efficacy of LVR coil treatment in homogeneous emphysema, which represents a large number of patients usually excluded from other surgical and bronchoscopic lung volume reduction treatment options.
In conclusion, this study provides multicentre evidence for the feasibility, procedural safety and efficacy of LVR coil treatment in patients with both heterogeneous and homogeneous emphysema. Further studies are underway to confirm efficacy in long-term randomised trials. Additional studies are needed to improve the understanding of the predictive factors of response in order to better select the responders to LVR coil treatment.
Footnotes
Contributors: GD, KK and DS operated as a writing committee and drafted the manuscript until it reached its final form. GD, KK, MH, FS, RK, C-HM, CW, SB, WG, FJFH, JH and D-JS all participated as investigators in this trial, recruited patients, performed the treatments and commented on the manuscript and its revisions. EMvR wrote the sections on CT and performed all the imaging analysis.
Competing interests: The sponsor designed the trial together with GD and D-JS, and facilitated monitoring of safety and independent audit, collection and storage of data. The authors had full access to all the data in the study and had final responsibility to submit for publication. The hospitals of the local PIs (GD, MH, FS, RK, C-HM, CW, SB, WG, FJFH, JH and D-JS) were reimbursed for study-related costs. KK received and MH receives consultancy fees from PneumRx. D-JS and GD are physician advisors to PneumRx and received travel reimbursements and speaker fees for educational sessions. EMvR has no competing interest.
Ethics approval: Ethics approval was obtained from the ethics committee at each site.
Patient consent: All subjects gave written informed consent.
References
- 1.Shah PL, Herth FJ. Current status of bronchoscopic lung volume reduction with endobronchial valves. Thorax 2014;69:280–6.. [DOI] [PubMed] [Google Scholar]
- 2.Gasparini S, Zuccatosta L, Bonifazi M, et al. Bronchoscopic treatment of emphysema: state of the art. Respiration 2012;84:250–63. [DOI] [PubMed] [Google Scholar]
- 3.Fishman A, Martinez F, Naunheim K, et al. A randomized trial comparing lung-volume-reduction surgery with medical therapy for severe emphysema. N Engl J Med 2003;348:2059–73. [DOI] [PubMed] [Google Scholar]
- 4.Criner GJ, Cordova F, Sternberg AL, et al. The NETT: part I. Lessons learned about emphysema. Am J Respir Crit Care Med 2011;184:763–70. [DOI] [PubMed] [Google Scholar]
- 5.Meyers BF, Patterson GA. Chronic obstructive pulmonary disease. 10: Bullectomy, lung volume reduction surgery, and transplantation for patients with chronic obstructive pulmonary disease. Thorax 2003;58:634–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sciurba FC, Ernst A, Herth FJ, et al. A randomized study of endobronchial valves for advanced emphysema. N Engl J Med 2010;363:1233–44. [DOI] [PubMed] [Google Scholar]
- 7.Herth FJ, Noppen M, Valipour A, et al. Efficacy predictors of lung volume reduction with Zephyr valves in a European cohort. Eur Respir J 2012;39:1334–42. [DOI] [PubMed] [Google Scholar]
- 8.Herth FJ, Eberhardt R, Gompelmann D, et al. Radiological and clinical outcomes of using Chartis to plan endobronchial valve treatment. Eur Respir J 2013;41:302–8. [DOI] [PubMed] [Google Scholar]
- 9.Herth FJ, Eberhard R, Gompelmann D, et al. Bronchoscopic lung volume reduction with a dedicated coil: a clinical pilot study. Ther Adv Respir Dis 2010;4:225–31. [DOI] [PubMed] [Google Scholar]
- 10.Slebos DJ, Klooster K, Ernst A, et al. Bronchoscopic lung volume reduction coil treatment of patients with severe heterogeneous emphysema. Chest 2012;142:574–82. [DOI] [PubMed] [Google Scholar]
- 11.Donohue JF. Minimal clinically important differences in COPD lung function. COPD 2005;2:111–24. [DOI] [PubMed] [Google Scholar]
- 12.Hartman JE, Ten Hacken NH, Klooster K, et al. The minimal important difference for residual volume in patients with severe emphysema. Eur Respir J 2012;40:1137–41. [DOI] [PubMed] [Google Scholar]
- 13.Puhan MA, Chandra D, Mosenifar Z, et al. The minimal important difference of exercise tests in severe COPD. Eur Respir J 2011;37:784–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Jones PW. St George's respiratory questionnaire: MCID. COPD 2005;2:75–9. [DOI] [PubMed] [Google Scholar]
- 15.Jones PW, Quirk FH, Baveystock CM, et al. A self-complete measure of health status for chronic airflow limitation. The St George's Respiratory Questionnaire. Am Rev Respir Dis 1992;145:1321–7. [DOI] [PubMed] [Google Scholar]
- 16.Miller MR, Crapo R, Hankinson J, et al. General considerations for lung function testing. Eur Respir J 2005;26:153–61. [DOI] [PubMed] [Google Scholar]
- 17.ATS Committee on Proficiency Standards for Clinical Pulmonary Function Laboratories. ATS statement: guidelines for the six-minute walk test. Am J Respir Crit Care Med 2002;166:111–7. [DOI] [PubMed] [Google Scholar]
- 18.van Rikxoort EM, de Hoop B, Viergever MA, et al. Automatic lung segmentation from thoracic computed tomography scans using a hybrid approach with error detection. Med Phys 2009;36:2934–47. [DOI] [PubMed] [Google Scholar]
- 19.Shah PL, Zoumot Z, Singh S, et al. Endobronchial coils for the treatment of severe emphysema with hyperinflation (RESET): a randomised controlled trial. Lancet Respir Med 2013;1:233–40. [DOI] [PubMed] [Google Scholar]
- 20.Shah PL, Slebos DJ, Cardoso PF, et al. Bronchoscopic lung-volume reduction with Exhale airway stents for emphysema (EASE trial): randomised, sham-controlled, multicentre trial. Lancet 2011;378:997–1005. [DOI] [PubMed] [Google Scholar]
- 21.Deslee G, Barbe C, Bourdin A, et al. Cost-effectiveness of lung volume reduction coil treatment in emphysema: STIC REVOLENS. Rev Mal Respir 2012;29:1157–64. [DOI] [PubMed] [Google Scholar]
- 22.Washko GR, Martinez FJ, Hoffman EA, et al. Physiological and computed tomographic predictors of outcome from lung volume reduction surgery. Am J Respir Crit Care Med 2010;181:494–500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Cooper CB. The connection between chronic obstructive pulmonary disease symptoms and hyperinflation and its impact on exercise and function. Am J Med 2006;119:21–31. [DOI] [PubMed] [Google Scholar]
- 24.Garcia-Rio F, Lores V, Mediano O, et al. Daily physical activity in patients with chronic obstructive pulmonary disease is mainly associated with dynamic hyperinflation. Am J Respir Crit Care Med 2009;180:506–12. [DOI] [PubMed] [Google Scholar]
- 25.Baraldo S, Turato G, Saetta M. Pathophysiology of the small airways in chronic obstructive pulmonary disease. Respiration 2012;84:89–97. [DOI] [PubMed] [Google Scholar]
- 26.Stoller JK, Gildea TR, Ries AL, et al. Lung volume reduction surgery in patients with emphysema and alpha-1 antitrypsin deficiency. Ann Thorac Surg 2007;83(Suppl 1):241–51. [DOI] [PubMed] [Google Scholar]
- 27.Mahler DA, Faryniarz K, Tomlinson D, et al. Impact of dyspnea and physiologic function on general health status in patients with chronic obstructive pulmonary disease. Chest 1992;102:395–401. [DOI] [PubMed] [Google Scholar]
- 28.Naunheim KS, Wood DE, Mohsenifar Z, et al. Long-term follow-up of patients receiving lung-volume-reduction surgery versus medical therapy for severe emphysema by the National Emphysema Treatment Trial Research Group. Ann Thorac Surg 2006;82:431–43. [DOI] [PubMed] [Google Scholar]
- 29.Snell G, Herth FJ, Hopkins P, et al. Bronchoscopic thermal vapour ablation therapy in the management of heterogeneous emphysema. Eur Respir J 2012;39: 1326–33. [DOI] [PubMed] [Google Scholar]
- 30.Herth FJ, Gompelmann D, Stanzel F, et al. Treatment of advanced emphysema with emphysematous lung sealant (AeriSeal(R)). Respiration 2011;82:36–45. [DOI] [PubMed] [Google Scholar]