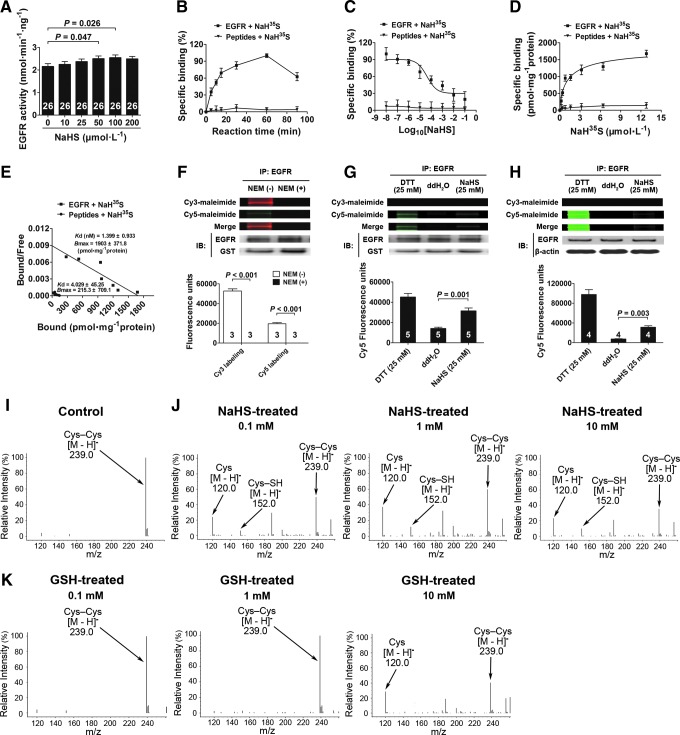
FIG. 6.
H2S directly activates EGFR and breaks its disulfide bond. (A) EGFR recombinant protein was treated with increasing concentrations of NaHS for 60 min, and the EGFR activity was detected. (B–E) NaH35S incubated with EGFR for different time scales (0–90 min) at 37°C (B) (n=6). Homologous competition curve for NaH35S binding against EGFR in vitro. Data were transformed into percentage of specific binding (%) and described by the one-site model (C) (n=6). A saturation binding experiment was performed at 37°C using increasing concentrations (0–12.8 μM) of NaH35S (D) (n=6). Scatchard analysis from saturation experiment gave the Kd and Bmax (E) (n=6). Peptides were used as a negative control. (F) Pretreated with NEM for 2 h to cover all -SH groups of the Cys residues in the intracellular kinase domain of EGFR. Cy3 and Cy5 labeled EGFR with fluorophore-conjugated maleimide. A representative fluorescence image was shown. The fluorescence intensities were quantified and normalized to intensities of the corresponding EGFR bands. (G) Differential Cys labeling of the recombination EGFR proteins treated with DTT, ddH2O, and NaHS, as done in (F). (H) Differential Cys labeling of the EGFR immunoprecipitated from cell lysate treated with DTT, ddH2O, and NaHS, as done in (F). (I–K) Mass spectrometry spectrum showing cleavage of the disulfide bond in Cys–Cys with treatment of NaHS and GSH. An S-sulfhydrated intermediate (Cys–SH) was identified during the process of the cleavage of the disulfide bond. Data in the graphs are means±SEM. Cys, cysteine; GSH, glutathione; NEM, N-ethylmaleimide. See Supplementary Figures S7 and S8 for more information.