Abstract
Background and purpose
Patients with epilepsy and malformations of cortical development (MCDs) are at high risk for language and other cognitive impairment. Specific impairments, however, are not well correlated with the extent and locale of dysplastic cortex; such findings highlight the relevance of aberrant cortico-cortical interactions, or connectivity, to the clinical phenotype. The goal of this study was to determine the independent contribution of well-described white matter pathways to language function in a cohort of pediatric patients with epilepsy.
Materials and methods
Patients were retrospectively identified from an existing database of pediatric epilepsy patients with the following inclusion criteria: 1. diagnosis of MCDs, 2. DTI performed at 3 T, and 3. language characterized by a pediatric neurologist. Diffusion Toolkit and Trackvis (http://www.trackvis.org) were used for segmentation and analysis of the following tracts: corpus callosum, corticospinal tracts, inferior longitudinal fasciculi (ILFs), inferior fronto-occipital fasciculi (IFOFs), uncinate fasciculi (UFs), and arcuate fasciculi (AFs). Mean diffusivity (MD) and fractional anisotropy (FA) were calculated for each tract. Wilcoxon rank sum test (corrected for multiple comparisons) was used to assess potential differences in tract parameters between language-impaired and language-intact patients. In a separate analysis, a machine learning algorithm (random forest approach) was applied to measure the independent contribution of the measured diffusion parameters for each tract to the clinical phenotype (language impairment). In other words, the importance of each tract parameter was measured after adjusting for the contribution of all other tracts.
Results
Thirty-three MCD patients were included (age range: 3–18 years). Twenty-one patients had intact language, twelve had language impairment. All tracts were identified bilaterally in all patients except for the AF, which was not identified on the right in 10 subjects and not identified on the left in 11 subjects. MD and/or FA within the left AF, UF, ILF, and IFOF differed between language-intact and language-impaired groups. However, only parameters related to the left uncinate, inferior fronto-occipital, and arcuate fasciculi were independently associated with the clinical phenotype.
Conclusions
Scalar metrics derived from the left uncinate, inferior fronto-occipital, and arcuate fasciculi were independently associated with language function. These results support the importance of these pathways in human language function in patients with MCDs.
Abbreviations: AF, arcuate fasciculus; BA, Broca's area; DWI, diffusion-weighted imaging; DTI, diffusion tensor imaging; FA, fractional anisotropy; IFOF, inferior fronto-occipital fasciculus; ILF, inferior longitudinal fasciculus; MCDs, malformations of cortical development; MD, mean diffusivity; UF, uncinate fasciculus; WA, Wernicke's area
Keywords: Epilepsy, Malformations of cortical development, Language, Tractography, Connectivity, Uncinate fasciculus, Arcuate fasciculus, Inferior fronto-occipital fasciculus
Highlights
-
•
Language phenotype was modeled based on metrics derived from whole-brain tractography.
-
•
We used machine learning to account for the influence of all other variables.
-
•
Metrics related to the left UF, IFOF, and AF were independently related to language.
-
•
The machine learning algorithm accurately classified individual language function.
1. Introduction
Patients with epilepsy and malformations of cortical development (MCDs) are at high risk for language and other cognitive impairment. Specific deficits, however, are not well correlated with the extent and locale of dysplastic cortex, highlighting the import of aberrant cortico-cortical interaction, or connectivity, to the clinical phenotype (Krsek et al., 2009). Given that surgical resection of focal epileptogenic lesions has become a frequent choice in management of the patient with intractable focal seizures, delineation of those pathways crucial to language function would be of great potential value to optimal patient management.
With the advent of diffusion-weighted imaging, the microstructural properties of a tissue of interest can be non-invasively probed at a spatial scale that is otherwise unattainable using even the most advanced structural MR techniques. Diffusion tensor imaging (DTI) is a variation on the theme of DWI which quantifies water motion in three orthogonal dimensions and, therefore, is better able to capture the anisotropic tendencies of diffusion in highly organized tissues such as cerebral white matter (Basser et al., 1994). Diffusion tractography is an extension of DTI in which the directional tendencies of water diffusion are used to create three dimensional representations of white matter tracts based on their structural coherence (Lee et al., 2005; Melhem et al., 2002). In many instances, the functional role of the constructed pathways is at least in part known, which enables assessment of brain parenchymal abnormalities in terms of functional systems (Catani et al., 2008; Vishwas et al., 2010).
Numerous scalar metrics can be derived from the tensor and used to probe the microstructural character of individual white matter pathways; the most commonly referenced are MD and FA. MD provides a measure of overall incoherent motion within a voxel without regard for direction and reflects tissue organization at the cellular level (Chenevert et al., 2000). Increased MD is a common manifestation of white matter pathology of diverse etiology (Vishwas et al., 2010; Lochner et al., 2012; Della Nave et al., 2008). By contrast, FA provides a measure of the degree to which a single direction of water motion dominates overall diffusivity in a voxel. As such, FA has been shown to be a relatively robust measure of white matter integrity (Lee et al., 2012; Beppu et al., 2012; Qiu et al., 2010; Ptak et al., 2003; Deppe et al., 2007).
Abnormalities within several white matter pathways have been reported in patients with language dysfunction (Harvey et al., 2013; McDonald et al., 2008; Mills et al., 2013). However, great potential exists to detect indirect associations (epiphenomena) between a proposed biomarker and a particular cognitive function, particularly in patient populations whose cerebral connectivity and brain function are both extensively abnormal. Furthermore, the ability to apply quantitative information garnered from such imaging techniques toward management of an individual patient has, to date, proved elusive. We sought to use a random forest approach, a form of machine learning, to overcome these two commonly encountered challenges in quantitative imaging.
Random forests are an ensemble learning method for classification that operate by constructing a multitude of decision trees at training time and outputting the class that is the mode of the classes output by individual trees (Breiman, 2001). At a fundamental level, this approach is based on bootstrap aggregating, or bagging, in which numerous models are fitted using individual bootstrap samples then combined by averaging. A strength of this particular technique is its ability to estimate the independent contribution of an individual variable while accounting for the contribution of all other variables. This estimate of variable importance is accomplished by measuring the error for each data point over the forest compared to that error which results when that variable is negated during bagging. Another strength of the random forest algorithm lies in its ability to provide an unbiased measure of classification accuracy. During each bootstrap iteration, approximately one third of the cohort is omitted at random from the training set – this omitted portion of the dataset is considered “out-of-bag” – classification of out-of-bag individuals is then predicted based on the generated model.
The goals of this study were two-fold: 1. to quantify the independent contribution of well-described white matter pathways to language function in a cohort of pediatric patients with epilepsy and 2. to measure the accuracy of the random forest algorithm with respect to classification of language phenotype in an individual patient.
2. Methods
This health insurance portability and accountability act-compliant study was approved by the local institutional review board. Patients were identified retrospectively from an existing database of pediatric patients undergoing clinical evaluation as part of the institutional multidisciplinary epilepsy work-group. The following inclusion criteria were applied: 1. pediatric age group (≤18 years), 2. diagnosis of malformation of cortical development established by MRI, 3. MRI of the brain performed at 3 T, including DTI, and 4. language development characterized by a pediatric neurologist. Refinements to the above-defined population were based on the following exclusion criteria: 1. motion or other degradation to image quality and 2. increase in confidence in the clinical determination of language delay; patients younger than 3 years of age were also excluded.
Patients were divided initially into three groups based on characterization of their language development by a pediatric neurologist: 1. intact: age-appropriate, 2. mild-to-moderate impairment: delayed by comparison to peers (either expressive or receptive), and 3. profound impairment: absence of oral language. This three-point scale was selected as it has been shown to provide both a clinically meaningful and a reproducible estimate of language function (Im et al., 2014). Twenty-one MCD patients had intact language, 9 mild-to-moderate impairment, and 3 profound impairment.
2.1. Magnetic resonance imaging
All imaging was performed on two 3 Tesla magnets (Siemens, Tim Trio, Erlangen, Germany). The following sequences were obtained: 1. sagittal magnetic preparation rapid acquisition gradient echo (TR/TE: 2530 ms/3.39 ms; 1 acquisition; flip: 7°, inversion time: 1100 ms; acceleration: 2; voxel (mm): 1 × 1 × 1), 2. axial fast spin echo T2-weighted (FSE; TR/TE: 11,730 ms/89 ms; 2 acquisitions; flip: 120°; acceleration: 2; voxel (mm): 0.6 × 0.4 × 2.5), 3. axial fluid attenuation inversion recovery (FLAIR; TR/TE: 9000 ms/137 ms; 1 acquisition; flip: 150°; FOV: 22 cm; voxel (mm): 0.7 × 0.7 × 4), and 4. axial single-shot echo planar imaging DTI (EPI; TR/TE (ms): 7000/90; flip: 90°; 1 acquisition; voxel (mm): 2 × 2 × 2). For DTI, 35 image sets were acquired, five without diffusion weighting (b0) and thirty with non-collinear diffusion-weighting gradients (b value: 1000 s/mm2). All images were visually inspected for artifacts, including subject motion.
2.2. Image processing and analysis
A single user experienced in tractography performed tract reconstruction, segmentation, and analysis. Maps of MD and FA were created using Diffusion Toolkit (http://www.trackvis.org). For each voxel, a tensor matrix was derived. After diagonalization of the matrix, eigenvalues were obtained and MD and FA were quantified for each pixel according to standard equations (Basser and Pierpaoli, 1996). Diffusion Toolkit (http://www.trackvis.org) was also used for deterministic tract reconstruction using a Fiber Association by Continuous Tracking algorithm (FACT; 35 degree angular threshold). A DWI mask was used to remove cerebrospinal fluid, a process which has been shown to effectively prevent spurious tract reconstruction (Vishwas et al., 2010). Trackvis (http://www.trackvis.org) was then used for segmentation and analysis of the following major commissural, projection, and intra-hemispheric association pathways: 1. corpus callosum, 2. corticospinal tracts, 3. arcuate fasciculi, 4. inferior longitudinal fasciculi, 5. inferior fronto-occipital fasciculi, and 6. uncinate fasciculi. Regions of interest for tract segmentation were placed manually on the color FA maps cross-referenced to the b0 images according to previously described methods (Wakana et al., 2007). Mean MD and mean FA were then calculated for each identifiable tract.
2.3. Data analysis and statistics
Statistical testing was performed using R statistical software package, version 3.0.2 (R Foundation for Statistical Computing, Vienna, Austria). Wilcoxon rank sum test (corrected for multiple comparisons) was used to assess potential differences in tract parameters between language-impaired and language-intact patients (alpha: 0.05 corrected).
In a separate analysis, a machine learning algorithm was used to quantify the independent contribution of the measured diffusion parameters for each tract to the clinical phenotype (language impairment). In other words, the importance of each tract parameter was measured after adjusting for the contribution of all other tracts. This analysis was accomplished using a random forest approach, which has been previously described in detail (Breiman, 2001). In short, to measure the independent contribution of an individual variable, the error for each data point was recorded over the forest and compared to that error which resulted after that variable had been negated during bagging; variable importance is presented normalized to the standard deviation of these differences.
Classification accuracy was also estimated internally by the random forest algorithm, as follows: During each bootstrap, approximately one-third of the cohort was omitted at random from the training set. This omitted portion of the dataset is considered “out-of-bag”. Classification of out-of-bag individuals was then predicted based on the fitted model. The out-of-bag error was calculated as the number of individuals incorrectly classified divided by the total number of individuals out-of-bag. Sensitivity, specificity, and positive and negative predictive values were also calculated for the language classification of out-of-bag individuals as predicted by the forest. The random forest algorithm was first performed allowing access to all diffusion metrics from all white matter pathways, as well as to age and gender. It was repeated for a reduced model, during which the algorithm was allowed access only to variables deemed to make an independent contribution (as determined above) to the classification of language function.
3. Results
3.1. Patients
Imaging was performed from January 2009 to August 2011. 33 patients (age range: 3–18 years; median: 10 years) comprised the final study group. Malformations of cortical development included: polymicrogyria (n = 15), focal cortical dysplasia (n = 13), schizencephaly (n = 4) and gray matter heterotopias (n = 1). Tract reconstruction and analysis identified all expected tracts in all subjects, with the exception of the arcuate fasciculus which was not identified on the left in eleven subjects and not identified on the right in ten subjects. To be specific, the arcuate was deemed to be absent when no streams could be visualized at tractography to directly connect the frontal and temporal lobes. As such, in addition to MD and FA measured from the arcuate fasciculus, its presence/absence was included as a variable in the model. Twenty-one patients had intact language; twelve had impaired language (9 mild-to-moderate; 3 profound). All patients (and their parents) were native English speakers.
3.2. Diffusion metrics and variable importance
Population averaged diffusion metrics measured from individual white matter pathways are presented in Table 1. After correcting for multiple comparisons, MD and/or FA within several left-sided tracts (left AF, UF, ILF, and IFOF) differed significantly between the language-intact and language-impaired groups (Table 1). However, only metrics related to the left arcuate, inferior fronto-occipital, and uncinate fasciculi were associated with the clinical phenotype of language impairment when accounting for the contribution of all other tracts (Fig. 1). For the left arcuate, MD, FA as well as the absence/presence of the pathway all contributed to language function. For the left uncinate and inferior fronto-occipital fasciculi, both of which were identified in all patients, MD and FA were both important contributors to the clinical phenotype. Out-of-bag error was estimated at 3.03% over the forest. Sensitivity, specificity, NPV and PPV of the forest-predicted classification for language function were all greater than 95% (Table 2). Out-of-bag error and diagnostic accuracy were all unchanged when training and classification were performed using only variables related to the left AF, IFOF, and UF.
Table 1.
Summary of mean diffusion metrics by individual white matter tract. In this case, the p-value measures the likelihood that the magnitude of difference (or greater) between the normal and abnormal language populations might be observed if the null hypothesis was true (adjusted for multiple comparisons).
| Variable | Normal language (N = 21) |
Abnormal language (N = 12) |
p-Value | ||
|---|---|---|---|---|---|
| Mean | Standard dev | Mean | Standard dev | ||
| Age (years) | 11.33333 | 4.31663 | 8.75 | 5.61046 | >0.99 |
| CC_MD | 0.00087 | 0.00008 | 0.00089 | 0.00007 | >0.99 |
| CC_FA | 0.60851 | 0.04542 | 0.55348 | 0.08094 | 0.3105 |
| Left CSP_MD | 0.00077 | 0.00003 | 0.0008 | 0.00004 | 0.4669 |
| Left CSP_FA | 0.59926 | 0.03842 | 0.57653 | 0.05007 | >0.99 |
| Right CSP_MD | 0.00076 | 0.00003 | 0.0008 | 0.00005 | 0.9959 |
| Right CSP_FA | 0.5975 | 0.04471 | 0.59278 | 0.05036 | >0.99 |
| Left AF_MD | 0.00079 | 0.00005 | 0.00099 | 0.00009 | <0.0023 |
| Left AF_FA | 0.50591 | 0.03796 | 0.40714 | 0.1173 | <0.0023 |
| Right AF_MD | 0.00079 | 0.00004 | 0.0009 | 0.00011 | 0.3726 |
| Right AF_FA | 0.495 | 0.03288 | 0.40814 | 0.1074 | 0.6072 |
| Left ILF_MD | 0.00089 | 0.00008 | 0.00093 | 0.00009 | >0.99 |
| Left ILF_FA | 0.48974 | 0.05185 | 0.42366 | 0.08636 | 0.0483 |
| Right ILF_MD | 0.00086 | 0.00007 | 0.00093 | 0.00009 | 0.4232 |
| Right ILF_FA | 0.47922 | 0.06934 | 0.43784 | 0.06716 | >0.99 |
| Left IFOF_MD | 0.00082 | 0.00004 | 0.00108 | 0.00018 | <0.0023 |
| Left IFOF_FA | 0.5756 | 0.03127 | 0.40371 | 0.09905 | <0.0023 |
| Right IFOF_MD | 0.00085 | 0.00005 | 0.0009 | 0.0001 | >0.99 |
| Right IFOF_FA | 0.53205 | 0.05223 | 0.48369 | 0.07525 | >0.99 |
| Left UF_MD | 0.00086 | 0.00004 | 0.00098 | 0.00005 | <0.0023 |
| Left UF_FA | 0.50771 | 0.03591 | 0.36668 | 0.06277 | <0.0023 |
| Right UF_MD | 0.00087 | 0.00007 | 0.00095 | 0.00009 | 0.1449 |
| Right UF_FA | 0.48541 | 0.03813 | 0.4632 | 0.04536 | >0.99 |
MD: mean diffusivity (units of 10−3 mm2 s−1); FA: fractional anisotropy; CC: corpus callosum; CSP: corticospinal tract; AF: arcuate fasciculus; ILF: inferior longitudinal fasciculus; IFOF: inferior fronto-occipital fasciculus; UF: uncinate fasciculus.
Fig. 1.
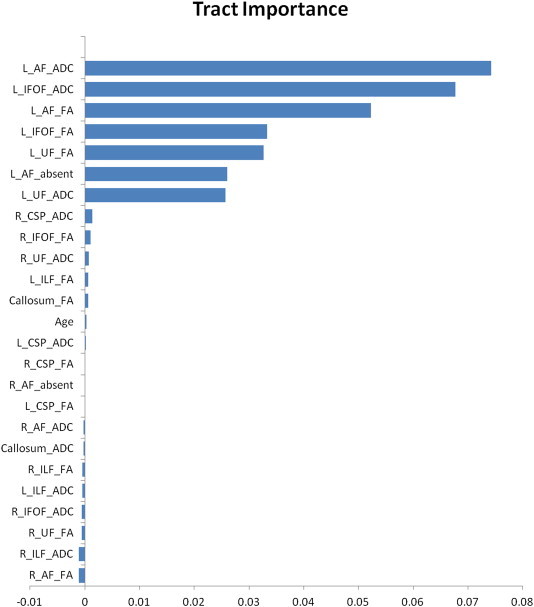
Importance of scalar metrics derived from individual white matter pathways depicted by whole brain tractography. The independent contribution of an individual variable was estimated by measuring the error for each data point recorded over the forest and comparing it to that error which results after that variable is negated during bagging. Variable importance is presented normalized to the standard deviation of these differences.
Table 2.
Diagnostic performance of the random forest algorithm for prediction of language impairment.
| Diagnostic performance | 95% LCI | 95% UCI | |
|---|---|---|---|
| Sensitivity (%) | 100 | 67.9 | 100 |
| Specificity (%) | 95.4 | 75.1 | 99.7 |
| PPV (%) | 91.6 | 59.8 | 99.6 |
| NPV (%) | 100 | 80.8 | 100 |
LCI: lower limit of the 95% confidence interval; UCI: upper limit of the 95% confidence interval; PPV: positive predictive value; NPV: negative predictive value.
4. Discussion
Using a machine learning approach, we report two main findings in a cohort of pediatric patients with malformations of cortical development: 1. diffusion metrics derived from the left uncinate, inferior fronto-occipital, and arcuate fasciculi were independently associated with language function and 2. individual patient language function was accurately classified by applying the machine learning algorithm to variables derived from whole brain tractography.
Although incompletely understood, the fluent comprehension and production of language is best conceptualized as an emergent property that results from complex interactions between distributed cortical regions across the cerebrum. Traditionally, it was thought to be predicated primarily upon Wernicke's (WA) and Broca's (BA) areas, located in the left posterior temporal and left inferior frontal lobes respectively, interacting via the arcuate fasciculus. However, current understanding suggests that WA and BA are but a part of a richly-interconnected, large-scale language network that extends to additional frontal, parietal, and temporal association areas in both hemispheres (Turken and Dronkers, 2011). A dual stream architecture of white matter pathways that promote such cortico-cortical interaction, and therefore subserve language function, has been proposed (Hickok and Poeppel, 2004). In general terms, the ventral stream is believed to link phonemic information with conceptual knowledge. The pathways that form the anatomic basis for this “stream” remain the subject of debate; roles for the uncinate, inferior longitudinal, and inferior fronto-occipital fasciculi have been suggested (Dick and Tremblay, 2012). Specific functional attributes of the dorsal stream are less well documented, but generally thought to involve the linkage between auditory and motor representations (Hickok and Poeppel, 2004). The arcuate/superior longitudinal fasciculus is generally considered to form the anatomic basis of the dorsal stream (Ellmore et al., 2009).
We observed an independent association of metrics derived from the left uncinate, inferior fronto-occipital, and arcuate fasciculi with language dysfunction in a cohort of pediatric patients with malformations of cortical development. These findings lend strong support to the idea that the AF, IFOF, and UF contribute significantly to those association fibers that form the connectional underpinnings of the human language network. Interestingly, MD and FA of these pathways both added value with respect to the predictive capacity of the model. This observation is consistent with the idea that each metric probes different aspects of tissue microstructure and suggests that they have distinct (or at least non-identical) physiologic implications. By contrast, metrics related to the left inferior longitudinal fasciculus were not associated with the clinical phenotype when accounting for the contribution made by other white matter pathways.
We also found that individual language impairment could be accurately predicted by a machine-learning algorithm applied to variables derived from whole brain tractography. Despite the extensive literature on quantitative imaging and its functional significance at the population level, clinical applications of these techniques remain few and far between (Jeong et al., 2014; Radhakrishnan et al., 2011; Powell et al., 2005; Tiwari et al., 2011). This shortcoming reflects, to a large degree, the relatively large inter-individual variation and the resultant difficulty in assigning a binary outcome (e.g., normal vs abnormal) to such continuous variables. Our findings suggest that machine learning is a potential mechanism by which quantitative imaging data could be translated into clinically relevant information in an individual epilepsy patient. Furthermore, the fact that the predictive capacity of the model was maintained when the software was given access only to metrics related to the left AF, IFOF, and UF lends further support to the idea that these particular pathways play a dominant role in the emergence of language function.
At the population level, abnormal microstructural properties of association pathways in the left hemisphere have been previously reported both in patients with localization related epilepsy (McDonald et al., 2008; Kucukboyaci et al., 2012; Govindan et al., 2008; Kim et al., 2011) and in patients with malformations of cortical development (Bernal et al., 2010; Munakata et al., 2006). Studies regarding the relevance of these abnormalities to language in the epilepsy population are relatively rare; however, in a small series of patients with congenital bilateral perisylvian syndrome, Saporta et al. observed a more severe language phenotype in patients with no identifiable arcuate fasciculus (Saporta et al., 2011). Similarly, McDonald et al. demonstrated a direct relationship between language, as measured by the Boston Naming Test, and diffusion properties within the left AF, UF and IFOF in patients with temporal lobe epilepsy; the ILF was not analyzed (McDonald et al., 2008). The authors in this study used hierarchical regression to control for age and hippocampal volume but did not account for the contribution of other white matter pathways. Despite the paucity of data in patients with seizures, the relevance of connectivity in the left hemisphere, specifically the microstructural character of the left AF, IFOF and UF, to language function has been suggested in other neurodevelopmental disorders, including autism, Angelman syndrome, and global developmental delay (Catani et al., 2013; Gopal et al., 2012; Jeong et al., 2011; Nagae et al., 2012; Peters et al., 2011; Sundaram et al., 2008; Wilson et al., 2011). Our work not only is consistent with this body of literature, but also significantly strengthens the evidence that the left AF, IFOF, and UF support connectivity in the language network in pediatric patients with localization related epilepsy.
Results supporting a role for the left ILF in human language have been less consistent. Mills et al. observed a relationship between white matter integrity of the left ILF and morphologic accuracy during a spoken narrative in patients with high functioning autism (Mills et al., 2013). In addition, Peters et al. observed a relationship between language and metrics derived from the left UF, AF, and ILF in patients with Angelman syndrome; the authors did not account for the contribution of other pathways to the clinical phenotype, however (Peters et al., 2011). By contrast, Harvey et al. demonstrated a relationship between structural integrity of the left UF, but not the ILF, and semantic control in a group of aphasic patients (Harvey et al., 2013). We found similar discrepant results in our study: whereas a statistical difference in left ILF FA was observed when comparing the language-impaired and language-normal study populations, metrics derived from the left ILF were not associated with the clinical phenotype when accounting for the contribution of other white matter pathways. The proximity of portions of the ILF to other important language pathways could potentially account for this discrepancy. In other words, anatomic abnormalities involving crucial language pathways in the left hemisphere might be expected to be more likely to also involve the ILF, potentially resulting in an indirect association, or epiphenomenon. Alternatively, the specific role(s) of the ILF in human language function may not be readily apparent during evaluation by a pediatric neurologist; rather, they may require detailed neuropsychologic testing to be detected. Findings by Mandonnet et al. suggest a final potential explanation (Mandonnet et al., 2007). In their study, subcortical stimulation during awake craniotomy at the level of the ILF elicited no language disturbance (Wilson et al., 2011; Mandonnet et al., 2007). Similarly, although they experienced transient language deficits, all patients recovered completely after resection of at least some part of the ILF. Taken together their results suggest that the ILF may not be indispensable for language. Yet consistent transient deficits after its resection in their study raise the possibility that the ILF may play a role in language that can be compensated after surgery or in other pathologic settings.
The use of machine learning to predict a clinical phenotype on the basis of quantitative imaging data has not, to our knowledge, been previously studied.
This study has several limitations. First, this is a study of a highly selected cohort of patients with localization-related epilepsy. As alluded to above, extrapolation of these results to patients with other types of CNS pathology may not be valid. Second, estimation of variable importance by the random forest algorithm can only dissociate the importance of those variables provided to it. In other words, the contribution of variables other than MD and FA within the included tracts, or even metrics derived from other parts of the brain entirely, could not be estimated. It is worth noting, however, that the algorithm was highly accurate, accounting for the vast majority of the variance in language phenotype with the provided variables. Third, functional assessment of language in this study was limited to the evaluation of a pediatric neurologist; this assessment was chosen in order to capture differences in language function with clear clinical relevance. Detailed neuropsychologic evaluation was not performed, but would be of great potential value to future studies. In particular, such an evaluation might allow the identification of specific domains of language dysfunction in each patient which could further elucidate functional sub-specialization within the language network. Finally, the inherent limitations of the tensor model should be acknowledged. The assumption that diffusivity in every voxel can be accurately described by a single ellipsoid is not always valid. The reliance of deterministic tracking algorithms on this model, therefore, compromises their ability to accurately characterize complex white matter architecture at the voxel/sub-voxel scale. Newer techniques, including high angular resolution diffusion imaging and diffusion spectrum imaging, are able to accommodate more complex probability displacement functions, allowing for more accurate tract construction (Kuo et al., 2008). This limitation, however, did not preclude in this case the accurate classification of language function by the machine learning algorithm.
5. Conclusion
In conclusion, we have used a machine learning approach to examine the relationship between structural connectivity in the left hemisphere and language dysfunction in pediatric epilepsy patients. In particular, we report the following: 1. diffusion metrics derived from the left uncinate, inferior fronto-occipital, and arcuate fasciculi were all independently associated with language function and 2. individual patient language function was accurately classified by applying the machine learning algorithm to variables derived from whole-brain tractography. These findings solidify the notion that the AF, IFOF, and UF form the connectional underpinnings of the human language network and, further, suggest that machine learning is a potential mechanism by which quantitative imaging data could be used to guide management in an individual patient with MCDs.
References
- Basser P.J., Mattiello J., LeBihan D. Estimation of the effective self-diffusion tensor from the NMR spin echo. Journal of Magnetic Resonance. Series B. 1994;103(3):247–254. doi: 10.1006/jmrb.1994.1037. 8019776 [DOI] [PubMed] [Google Scholar]
- Basser P.J., Pierpaoli C. Microstructural and physiological features of tissues elucidated by quantitative-diffusion-tensor MRI. Journal of Magnetic Resonance. Series B. 1996;111(3):209–219. doi: 10.1006/jmrb.1996.0086. 8661285 [DOI] [PubMed] [Google Scholar]
- Beppu T., Fujiwara S., Nishimoto H. Fractional anisotropy in the centrum semiovale as a quantitative indicator of cerebral white matter damage in the subacute phase in patients with carbon monoxide poisoning: correlation with the concentration of myelin basic protein in cerebrospinal fluid. Journal of Neurology. 2012;259(8):1698–1705. doi: 10.1007/s00415-011-6402-5. 22258479 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernal B., Rey G., Dunoyer C., Shanbhag H., Altman N. Agenesis of the arcuate fasciculi in congenital bilateral perisylvian syndrome: a diffusion tensor imaging and tractography study. Archives of Neurology. 2010;67(4):501–505. doi: 10.1001/archneurol.2010.59. 20385920 [DOI] [PubMed] [Google Scholar]
- Breiman L. Random forests. Machine Learning. 2001;45(1):5–32. [Google Scholar]
- Catani M., Mesulam M.M., Jakobsen E. A novel frontal pathway underlies verbal fluency in primary progressive aphasia. Brain. 2013;136(8):2619–2628. doi: 10.1093/brain/awt163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Catani M., Thiebaut de Schotten M. A diffusion tensor imaging tractography atlas for virtual in vivo dissections. Cortex; a Journal Devoted to the Study of the Nervous System and Behavior. 2008;44(8):1105–1132. doi: 10.1016/j.cortex.2008.05.004. 18619589 [DOI] [PubMed] [Google Scholar]
- Chenevert T.L., Stegman L.D., Taylor J.M. Diffusion magnetic resonance imaging: an early surrogate marker of therapeutic efficacy in brain tumors. Journal of the National Cancer Institute. 2000;92(24):2029–2036. doi: 10.1093/jnci/92.24.2029. 11121466 [DOI] [PubMed] [Google Scholar]
- Della Nave R., Ginestroni A., Tessa C. Brain white matter damage in Sca1 and Sca2. An in vivo study using voxel-based morphometry, histogram analysis of mean diffusivity and tract-based spatial statistics. Neuroimage. 2008;43(1):10–19. doi: 10.1016/j.neuroimage.2008.06.036. 18672073 [DOI] [PubMed] [Google Scholar]
- Deppe M., Duning T., Mohammadi S. Diffusion-tensor imaging at 3 T: detection of white matter alterations in neurological patients on the basis of normal values. Investigative Radiology. 2007;42(6):338–345. doi: 10.1097/01.rli.0000261935.41188.39. 17507803 [DOI] [PubMed] [Google Scholar]
- Dick A.S., Tremblay P. Beyond the arcuate fasciculus: consensus and controversy in the connectional anatomy of language. Brain: A Journal of Neurology. 2012;135(12):3529–3550. doi: 10.1093/brain/aws222. 23107648 [DOI] [PubMed] [Google Scholar]
- Ellmore T.M., Beauchamp M.S., O'Neill T.J., Dreyer S., Tandon N. Relationships between essential cortical language sites and subcortical pathways. Journal of Neurosurgery. 2009;111(4):755–766. doi: 10.3171/2009.3.JNS081427. 19374498 [DOI] [PubMed] [Google Scholar]
- Gopal S.P., Tiwari V.N., Veenstra A.L. Sensitive diffusion tensor imaging quantification method to identify language pathway abnormalities in children with developmental delay. Journal of Pediatrics. 2012;160(1):147–151. doi: 10.1016/j.jpeds.2011.06.036. 21839473 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Govindan R.M., Makki M.I., Sundaram S.K., Juhász C., Chugani H.T. Diffusion tensor analysis of temporal and extra-temporal lobe tracts in temporal lobe epilepsy. Epilepsy Research. 2008;80(1):30–41. doi: 10.1016/j.eplepsyres.2008.03.011. 18436432 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harvey D.Y., Wei T., Ellmore T.M., Hamilton A.C., Schnur T.T. Neuropsychological evidence for the functional role of the uncinate fasciculus in semantic control. Neuropsychologia. 2013;51(5):789–801. doi: 10.1016/j.neuropsychologia.2013.01.028. 23395830 [DOI] [PubMed] [Google Scholar]
- Hickok G., Poeppel D. Dorsal and ventral streams: a framework for understanding aspects of the functional anatomy of language. Cognition. 2004;92(1–2):67–99. doi: 10.1016/j.cognition.2003.10.011. 15037127 [DOI] [PubMed] [Google Scholar]
- Im K., Paldino M.J., Poduri A., Sporns O., Grant P.E. Altered white matter connectivity and network organization in polymicrogyria revealed by individual gyral topology-based analysis. Neuroimage. 2014;86:182–193. doi: 10.1016/j.neuroimage.2013.08.011. 23954485 [DOI] [PubMed] [Google Scholar]
- Jeong J.W., Asano E., Juhász C., Chugani H.T. Quantification of primary motor pathways using diffusion MRI tractography and its application to predict postoperative motor deficits in children with focal epilepsy. Human Brain Mapping. 2014 doi: 10.1002/hbm.22396. 24142581 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeong J.W., Sundaram S.K., Kumar A., Chugani D.C., Chugani H.T. Aberrant diffusion and geometric properties in the left arcuate fasciculus of developmentally delayed children: a diffusion tensor imaging study. AJNR. American Journal of Neuroradiology. 2011;32(2):323–330. doi: 10.3174/ajnr.A2382. 21183617 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim C.H., Chung C.K., Koo B.B., Lee J.M., Kim J.S., Lee S.K. Changes in language pathways in patients with temporal lobe epilepsy: diffusion tensor imaging analysis of the uncinate and arcuate fasciculi. World Neurosurgery. 2011;75(3–4):509–516. doi: 10.1016/j.wneu.2010.11.006. 21600505 [DOI] [PubMed] [Google Scholar]
- Krsek P., Maton B., Jayakar P. Incomplete resection of focal cortical dysplasia is the main predictor of poor postsurgical outcome. Neurology. 2009;72(3):217–223. doi: 10.1212/01.wnl.0000334365.22854.d3. 19005171 [DOI] [PubMed] [Google Scholar]
- Kucukboyaci N.E., Girard H.M., Hagler D.J., Jr. Role of frontotemporal fiber tract integrity in task-switching performance of healthy controls and patients with temporal lobe epilepsy. Journal of the International Neuropsychological Society: JINS. 2012;18(1):57–67. doi: 10.1017/S1355617711001391. 22014246 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuo L.W., Chen J.H., Wedeen V.J., Tseng W.Y. Optimization of diffusion spectrum imaging and q-ball imaging on clinical MRI system. Neuroimage. 2008;41(1):7–18. doi: 10.1016/j.neuroimage.2008.02.016. 18387822 [DOI] [PubMed] [Google Scholar]
- Lee E.C., Kwatra N.S., Vezina G., Khademian Z.P. White matter integrity on fractional anisotropy maps in encephalopathic neonates post hypothermia therapy with normal-appearing MR imaging. Pediatric Radiology. 2012 doi: 10.1007/s00247-012-2572-2. [DOI] [PubMed] [Google Scholar]
- Lee S.K., Kim D.I., Kim J. Diffusion-tensor MR imaging and fiber tractography: a new method of describing aberrant fiber connections in developmental CNS anomalies. Radiographics: A Review Publication of the Radiological Society of North America, Inc. 2005;25(1):53–65. doi: 10.1148/rg.251045085. 15653586 [DOI] [PubMed] [Google Scholar]
- Lochner C., Fouché J.P., du Plessis S. Evidence for fractional anisotropy and mean diffusivity white matter abnormalities in the internal capsule and cingulum in patients with obsessive–compulsive disorder. Journal of Psychiatry & Neuroscience: JPN. 2012;37(3):193–199. doi: 10.1503/jpn.110059. 22297066 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mandonnet E., Nouet A., Gatignol P., Capelle L., Duffau H. Does the left inferior longitudinal fasciculus play a role in language? A brain stimulation study. Brain: A Journal of Neurology. 2007;130(3):623–629. doi: 10.1093/brain/awl361. 17264096 [DOI] [PubMed] [Google Scholar]
- McDonald C.R., Ahmadi M.E., Hagler D.J. Diffusion tensor imaging correlates of memory and language impairments in temporal lobe epilepsy. Neurology. 2008;71(23):1869–1876. doi: 10.1212/01.wnl.0000327824.05348.3b. 18946001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Melhem E.R., Mori S., Mukundan G., Kraut M.A., Pomper M.G., van Zijl P.C. Diffusion tensor MR imaging of the brain and white matter tractography. AJR. American Journal of Roentgenology. 2002;178(1):3–16. doi: 10.2214/ajr.178.1.1780003. 11756078 [DOI] [PubMed] [Google Scholar]
- Mills B.D., Lai J., Brown T.T. White matter microstructure correlates of narrative production in typically developing children and children with high functioning autism. Neuropsychologia. 2013;51(10):1933–1941. doi: 10.1016/j.neuropsychologia.2013.06.012. 23810972 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Munakata M., Onuma A., Takeo K., Oishi T., Haginoya K., Iinuma K. Morphofunctional organization in three patients with unilateral polymicrogyria: combined use of diffusion tensor imaging and functional magnetic resonance imaging. Brain & Development. 2006;28(6):405–409. doi: 10.1016/j.braindev.2005.12.003. 16503392 [DOI] [PubMed] [Google Scholar]
- Nagae L.M., Zarnow D.M., Blaskey L. Elevated mean diffusivity in the left hemisphere superior longitudinal fasciculus in autism spectrum disorders increases with more profound language impairment. AJNR. American Journal of Neuroradiology. 2012;33(9):1720–1725. doi: 10.3174/ajnr.A3037. 22492573 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peters S.U., Kaufmann W.E., Bacino C.A. Alterations in white matter pathways in Angelman syndrome. Developmental Medicine and Child Neurology. 2011;53(4):361–367. doi: 10.1111/j.1469-8749.2010.03838.x. 21121904 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Powell H.W., Parker G.J., Alexander D.C. MR tractography predicts visual field defects following temporal lobe resection. Neurology. 2005;65(4):596–599. doi: 10.1212/01.wnl.0000172858.20354.73. 16116123 [DOI] [PubMed] [Google Scholar]
- Ptak T., Sheridan R.L., Rhea J.T. Cerebral fractional anisotropy score in trauma patients: a new indicator of white matter injury after trauma. AJR. American Journal of Roentgenology. 2003;181(5):1401–1407. doi: 10.2214/ajr.181.5.1811401. 14573445 [DOI] [PubMed] [Google Scholar]
- Qiu A., Oishi K., Miller M.I., Lyketsos C.G., Mori S., Albert M. Surface-based analysis on shape and fractional anisotropy of white matter tracts in Alzheimer's disease. PloS One. 2010;5(3):e9811. doi: 10.1371/journal.pone.0009811. 20339558 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Radhakrishnan A., James J.S., Kesavadas C. Utility of diffusion tensor imaging tractography in decision making for extratemporal resective epilepsy surgery. Epilepsy Research. 2011;97(1–2):52–63. doi: 10.1016/j.eplepsyres.2011.07.003. 21835594 [DOI] [PubMed] [Google Scholar]
- Saporta A.S., Kumar A., Govindan R.M., Sundaram S.K., Chugani H.T. Arcuate fasciculus and speech in congenital bilateral perisylvian syndrome. Pediatric Neurology. 2011;44(4):270–274. doi: 10.1016/j.pediatrneurol.2010.11.006. 21397168 [DOI] [PubMed] [Google Scholar]
- Sundaram S.K., Sivaswamy L., Makki M.I., Behen M.E., Chugani H.T. Absence of arcuate fasciculus in children with global developmental delay of unknown etiology: a diffusion tensor imaging study. Journal of Pediatrics. 2008;152(2):250–255. doi: 10.1016/j.jpeds.2007.06.037. 18206698 [DOI] [PubMed] [Google Scholar]
- Tiwari V.N., Jeong J.W., Asano E., Rothermel R., Juhasz C., Chugani H.T. A sensitive diffusion tensor imaging quantification method to detect language laterality in children: correlation with the Wada test. Journal of Child Neurology. 2011;26(12):1516–1521. doi: 10.1177/0883073811409225. 21652590 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turken A.U., Dronkers N.F. The neural architecture of the language comprehension network: converging evidence from lesion and connectivity analyses. Frontiers in Systems Neuroscience. 2011;5:1. doi: 10.3389/fnsys.2011.00001. 21347218 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vishwas M.S., Chitnis T., Pienaar R., Healy B.C., Grant P.E. Tract-based analysis of callosal, projection, and association pathways in pediatric patients with multiple sclerosis: a preliminary study. AJNR. American Journal of Neuroradiology. 2010;31(1):121–128. doi: 10.3174/ajnr.A1776. 19850763 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wakana S., Caprihan A., Panzenboeck M.M. Reproducibility of quantitative tractography methods applied to cerebral white matter. Neuroimage. 2007;36(3):630–644. doi: 10.1016/j.neuroimage.2007.02.049. 17481925 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson B.J., Sundaram S.K., Huq A.H. Abnormal language pathway in children with Angelman syndrome. Pediatric Neurology. 2011;44(5):350–356. doi: 10.1016/j.pediatrneurol.2010.12.002. 21481743 [DOI] [PMC free article] [PubMed] [Google Scholar]


