Abstract
Background:
The effects of nasal continuous positive airway pressure (CPAP) on insulin resistance (IR) in obstructive sleep apnea (OSA) are still under discussion especially in nondiabetics. Trials have found conflicting results in this regard.
Aims:
The study was to measure IR in nondiabetic patients with moderate to severe OSA and to evaluate the effect of nasal CPAP on IR in these patients.
Materials and Methods:
A total of 30 consecutively newly diagnosed patients with moderate to severe OSA was enrolled in the study. OSA was diagnosed by doing an overnight polysomnography. Plasma glucose and insulin levels were measured at baseline and after 1 month of CPAP treatment. IR was calculated by homeostasis model assessment (HOMA) method.
Results:
Of 30 OSA patients, 21 were males, and 9 were females. The mean age of the subjects was 49.9 years, and mean body mass index (BMI) was 29.33. All 30 patients had moderate to severe OSA with a mean apnea and hypopnea index (AHI) of 80.46/h. The Epworth sleepiness score (ESS) showed a significant change with 1 month of treatment with CPAP from baseline of 13 to 9.7 (P ≤ 0.0001). There was a significant reduction in fasting insulin levels from 21.75 to 19.39 (P = 0.009). There was a small fall in fasting glucose, but it was not significant. The HOMA IR also reduced from 5.78 to 4.82 which was significant (P = 0.024) without any significant change in BMI (P = 0.916). The HOMA IR did not showed any positive correlation with different variables of OSA severity, ESS (r = 0.156) (P = 0.410), AHI (r = 0.177) (P = 0.349), and percentage of test time <90% saturation (r = −0.296) (P = 0.112).
Conclusion:
Moderate to severe OSA is associated with an increase in IR and effective treatment with CPAP rapidly improves the insulin sensitivity in nondiabetic patients with OSA without any change in BMI.
Keywords: Continuous positive airway pressure, Insulin resistance, Obstructive sleep apnea
Introduction
Obstructive sleep apnea (OSA) is a condition characterized by occurrence of repeated episodes of upper airway obstruction and airflow cessation. It is associated with fragmented sleep, disruptive snoring, nocturnal hypoxemia, and daytime symptoms most commonly excessive sleepiness.[1] It approximately affects 2-4% of adult men and 1-2% of adult women.[2] It is well-recognized that OSA is associated with high cardiovascular morbidity and mortality.[3,4] A number of clinical features such as obesity, excessive daytime sleepiness (EDS), and insulin resistance (IR) are often but not invariably present in these patients.[5,6,7]
It is also recognized that many subjects with OSA have other features of metabolic syndrome which includes hypertension and hyperlipidemia,[8] which have been established as independent risk factors for vascular disease.[8,9] Obesity is generally regarded as a risk factor for both OSA and IR. However, factors other than obesity play a significant role in the development IR and metabolic disturbances in patients with OSA,[10,11,12] including sleep fragmentation, increased sympathetic activity, intermittent hypoxia, and decreased serum levels of adiponectin.[13,14]
Nasal continuous positive airway pressure (CPAP) is the treatment of choice in patients with moderate to severe OSA. It improves the quality of sleep, decreases EDS, and improves quality-of-life. Various studies have shown that treatment with nasal CPAP decreases the risk of cardiovascular morbidity and mortality in patients with moderate to severe OSA.[15] However, improvement in metabolic effects with CPAP treatment, e.g., improving IR are still under discussion especially in nondiabetics.[16,17,18,19,20] This study is designed to look for the effect of treatment with CPAP on IR in nondiabetic patients with moderate to severe OSA.
Materials and Methods
Subjects
This is a prospective interventional study that was conducted in Department of Pulmonary Medicine, St. John's Medical College Hospital, Bengaluru, Karnataka, India, which is a tertiary care center and a referral hospital. Thirty newly diagnosed patients with moderate to severe OSA and those who were willing to use nasal CPAP for 1 month were enrolled in the study. Subjects with diabetes mellitus, polycystic ovarian disease, acanthosis nigricans, thyroid dysfunction, current smoking history, steroid use in last 1 month were excluded from the study. Informed written consent was taken from the subjects and ethical clearance was obtained from the Ethical Committee.
Method of collection of data
Baseline data of all 30 subjects were collected which included age, sex, height, weight, presence of symptoms like snoring, EDS, choking during sleep, nocturia, apneic events. EDS was scored using Epworth sleepiness score (ESS). OSA was diagnosed by doing an overnight polysomnography. The apnea and hypopnea index (AHI), which is defined as the total number of apneas and hypopneas divided by total numbers of hours of sleep was calculated. Apnea was defined as the absence of airflow for more than 10 s. Hypopnea was defined as a reduction of airflow for more than 10 s with oxygen desaturation of ≥4%.
Patients with AHI >5/h were considered as diagnosed of OSA. Severity of OSA was decided by AHI. Patients with moderate to severe OSA (AHI ≥15) and meeting inclusion and exclusion criteria were enrolled in the study.
Samples of peripheral venous blood for measurement glucose and insulin were collected after overnight fasting and stored at −20°C until assay. CPAP titration was done in these patients to determine the pressure required to correct OSA. These patients were treated with nasal CPAP set at a fixed pressure determined from the titration study. After 1 month of treatment with nasal CPAP, fasting insulin and glucose were again obtained and stored at −20°C until assay.
Plasma glucose was measured by enzymatic method and plasma insulin by enzyme immunoassay using monoclonal antibody.
Insulin resistance was calculated by homeostasis model assessment (HOMA) method using:

Statistical analysis
Data analysis was done using SPSS for Windows version 13.0 (IBM SPSS Statistics). The significance of the difference in fasting glucose level, fasting insulin level, and HOMA IR before and after CPAP treatment was analyzed with Paired t-test. The baseline HOMA IR was correlated with the indices for severity of OSA-ESS, AHI, and percentage of test time <90% saturation using Pearson's coefficient of correlation (two-tailed). Statistical significance was set at P < 0.05.
Results
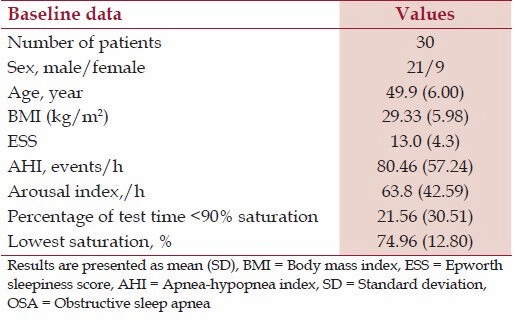
Thirty newly diagnosed patients with moderate to severe OSA who were willing to use nasal CPAP for 1 month meeting the inclusion and exclusion criterion were enrolled in the study. Among 30 OSA patients, 21 were males, and 9 were females. The mean age of the subjects was 49.9 years, and mean body mass index (BMI) was 29.33 [Table 1]. The mean ESS was 13.0. The study subjects had a mean AHI of 80.46/h and an arousal index of 63.8/h. The mean percentage of test time with saturation <90% was 21.56 and the lowest saturation was 74.96%.
Table 1.
Baseline data of patients with moderate to severe OSA
Metabolic parameters were determined at the time of diagnosis. The study subjects had a mean fasting insulin level of 21.75 μU/ml and fasting glucose of 100.36 mg/dl. The mean calculated HOMA IR was 5.78. Baseline HOMA IR was correlated with different variables of OSA severity using Pearson coefficient of correlation two-tailed. The HOMA IR did not showed any significant positive correlation with ESS (r = 0.156) (P = 0.41), AHI (r = 0.177) (P = 0.34) and percentage of test time <90% saturation (r = −0.296) (P = 0.11).
All the study subjects were titrated adequately. The mean CPAP pressure was 14 cmH2 O and ranged from 12 to 18 cmH2 O. During the titration, the AHI reduced from a mean of 80.46 to a mean of 5.7 and the saturation improved from 74.96% to 91.26% on room air. The patients were evaluated after 1 month of CPAP therapy. Mean CPAP use in our patients was 4.5 h/night, and the compliance of the use of nasal CPAP in our study was based on self-reporting. Patients were evaluated after 1 month of nasal CPAP treatment.
The ESS showed a significant change with treatment [Table 2] from 13 to 9.7 (P ≤ 0.001). There was a significant reduction in fasting insulin levels from 21.75 to 19.39 (P = 0.009). There was a small fall in fasting glucose from 100.36 to 98.7 (P = 0.71) but it was not significant. The HOMA IR also reduced from 5.78 to 4.82 [Figure 1] which was significant (P = 0.02). There was no significant change in BMI after 1 month of nasal CPAP use (P = 0.91).
Table 2.
Effect of nasal CPAP on different variables
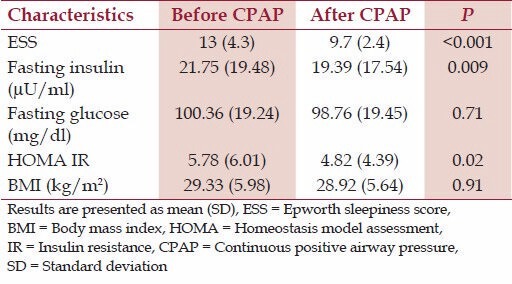
Figure 1.
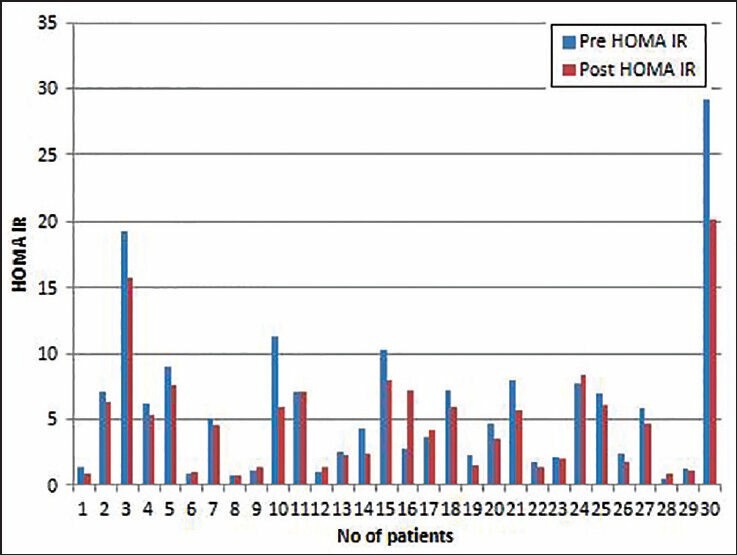
Data showing insulin responsiveness before (pre) and after (post) a month of nasal continuous positive airway pressure (CPAP) use in moderate to severe nondiabetic obstructive sleep apnea patients. Homeostasis model assessment insulin resistance method (HOMA IR) presented as mean (SD). There was a significant change in HOMA IR after a month of CPAP use (P = 0.02)
Discussion
Obstructive sleep apnea has so far been recognized more for its effects on quality-of-life than direct effects on health. Currently, there is a considerable interest in its association with cardiovascular and cerebrovascular diseases including hypertension, coronary artery disease (CAD), and noninsulin-dependent diabetes mellitus. Many patients with OSA are known to have coexisting risk factors for cardiovascular and cerebrovascular diseases which include central obesity, hypertension, dyslipidemia, and IR.[21]
Insulin resistance is the hallmark of type II diabetes and can eventually lead to the development of type 2 diabetes in 5.1 ± 1.4 years.[22] IR, as indicated by an impaired biological response to insulin and hence, a reduced insulin-mediated glucose disposal, has been implicated in the pathogenesis of the metabolic syndrome.[8] Resistance to insulin-mediated glucose disposal is present in 25% of nonobese individuals with normal oral glucose tolerance and the majority of patients with noninsulin-dependent diabetes.[16]
Obstructive sleep apnea exhibits pathophysiologic mechanisms that may potentially contribute to the development of IR, including autonomic activation, alterations in neuroendocrine function, direct effects of hypoxemia on glucose regulation, and release of proinflammatory cytokines such as interleukin-6 and tumor necrosis factor-alpha.[10] There are emerging data suggesting that IR may play an important role in the pathogenesis of hypertension and cardiovascular diseases.[23] It promotes atherosclerosis and increases the risk of myocardial infarction,[23] stroke, and peripheral vascular diseases. Population studies have shown that OSA is associated with IR, and the more severe the OSA, the greater the IR, independent of obesity.[10,11] Hence, treating OSA effectively with CPAP should bring down IR and thus, contribute to a reduction of cardiovascular risk in patients with OSA.
Our findings of IR are consistent with the finding of Barceló et al., who found HOMA IR of 4.3 among severe OSA patients with mean BMI of 32.[24] In the evaluation of IR, the gold standard is the euglycemic clamp method.[25] However, this method is invasive, expensive, and labor intensive. In our study, we have used HOMA IR index. The HOMA IR was proposed as a simple and inexpensive alternative to more sophisticated techniques.[26] The method derives an estimation of IR from the fasting blood glucose and corresponding fasting plasma insulin levels. It has been compared with other methods of measuring IR such as glucose clamp technique, intravenous glucose tolerance test, and frequently sampled intravenous glucose tolerance test.[27,28] Results have indicated that there is a close correlation between IR measured by HOMA and glucose clamp technique (r = −0.64, P < 0.0001). Due to its simplicity, HOMA is used in large clinical studies.[29]
We did not find any positive correlation of HOMA IR with ESS or AHI or percentage of test time <90% saturation. This may be due to the small sample size. It may also be due to fact that we included only moderately severe to severe OSA patients and hence were unable to show a correlation with severity. The other possibility is that IR attributable to OSA is small and constant and not related to the severity.
A study by Brooks et al. showed a tendency to improved insulin sensitivity after 4 months of CPAP treatment measured by hyperinsulinemic euglycemic clamp in severely obese with a mean BMI of 41.6 who were diabetic.[19] But in another study (measurements after 2 months) by Saarelainen et al. could not confirm this result in nondiabetic OSA patients with BMI of 34.4.[16] In this study, there was a trend to improvement in fasting insulin levels from 21.06 to 15.95 without any change in body weight. This study did not achieve statistical significance due to its small number. Variation in lifestyle like physical activity, diet, nicotine, alcohol consumption etc., can also affect IR. This effect may influence the results to a greater extent in a small population. Furthermore, if the reassessment of insulin sensitivity is done 2-4 months after onset of CPAP treatment, other factors influencing insulin sensitivity (weight, body fat distribution, treatment of concomitant diseases, changes in dietary behavior, smoking, alcohol consumption, physical activity) may affect IR.
In our study baseline, insulin was 21.75 which is comparable to the above study. Our sample size was relatively larger, and reassessment of IR was done 1 month after CPAP treatment. In such a short period, other confounders are less likely to affect IR. In our patients, HOMA IR and insulin levels significantly decreased after CPAP treatment despite no change in BMI. Hence, we assume that IR is increased in OSA independent of obesity and improves on treatment with CPAP.
Our study shows that in nondiabetics with moderate to severe OSA, there is an increased IR which may be one of the reasons that OSA is a risk factor for CAD. CPAP is known to lower blood pressure. Hence, identifying OSA and treating it adequately may be important in decreasing the risk to develop CAD. In patients with CAD, it may be important to look for OSA and treat it. It would also be interesting to know whether treating OSA long-term decreases the risk of CAD. The current study suggests that there is a rationale to do so.
Footnotes
Source of Support: Nil.
Conflict of Interest: None declared.
References
- 1.Douglas NJ, Polo O. Pathogenesis of sleep apnea/hypopnoea syndrome. Lancet. 1994;344:653–5. doi: 10.1016/s0140-6736(94)92088-5. [DOI] [PubMed] [Google Scholar]
- 2.Young T, Palta M, Dempsey J, Skatrud J, Weber S, Badr S. The occurrence of sleep-disordered breathing among middle-aged adults. N Engl J Med. 1993;328:1230–5. doi: 10.1056/NEJM199304293281704. [DOI] [PubMed] [Google Scholar]
- 3.Shahar E, Whitney CW, Redline S, Lee ET, Newman AB, Nieto FJ, et al. Sleep-disordered breathing and cardiovascular disease: Cross-sectional results of the Sleep Heart Health Study. Am J Respir Crit Care Med. 2001;163:19–25. doi: 10.1164/ajrccm.163.1.2001008. [DOI] [PubMed] [Google Scholar]
- 4.He J, Kryger MH, Zorick FJ, Conway W, Roth T. Mortality and apnea index in obstructive sleep apnea. Experience in 385 male patients. Chest. 1988;94:9–14. [PubMed] [Google Scholar]
- 5.Durán J, Esnaola S, Rubio R, Iztueta A. Obstructive sleep apnea-hypopnea and related clinical features in a population-based sample of subjects aged 30 to 70 year. Am J Respir Crit Care Med. 2001;163:685–9. doi: 10.1164/ajrccm.163.3.2005065. [DOI] [PubMed] [Google Scholar]
- 6.Tasali E, Ip MS. Obstructive sleep apnea and metabolic syndrome: Alterations in glucose metabolism and inflammation. Proc Am Thorac Soc. 2008;5:207–17. doi: 10.1513/pats.200708-139MG. [DOI] [PubMed] [Google Scholar]
- 7.Lam JC, Ip MS. An update on obstructive sleep apnea and the metabolic syndrome. Curr Opin Pulm Med. 2007;13:484–9. doi: 10.1097/MCP.0b013e3282efae9c. [DOI] [PubMed] [Google Scholar]
- 8.Reaven GM. Banting lecture 1988.Role of insulin resistance in human disease. Diabetes. 1988;37:1595–607. doi: 10.2337/diab.37.12.1595. [DOI] [PubMed] [Google Scholar]
- 9.Kannel WB. Some lessons in cardiovascular epidemiology from Framingham. Am J Cardiol. 1976;37:269–82. doi: 10.1016/0002-9149(76)90323-4. [DOI] [PubMed] [Google Scholar]
- 10.Punjabi NM, Polotsky VY. Disorders of glucose metabolism in sleep apnea. J Appl Physiol (1985) 2005;99:1998–2007. doi: 10.1152/japplphysiol.00695.2005. [DOI] [PubMed] [Google Scholar]
- 11.Ip MS, Lam B, Ng MM, Lam WK, Tsang KW, Lam KS. Obstructive sleep apnea is independently associated with insulin resistance. Am J Respir Crit Care Med. 2002;165:670–6. doi: 10.1164/ajrccm.165.5.2103001. [DOI] [PubMed] [Google Scholar]
- 12.Meslier N, Gagnadoux F, Giraud P, Person C, Ouksel H, Urban T, et al. Impaired glucose-insulin metabolism in males with obstructive sleep apnoea syndrome. Eur Respir J. 2003;22:156–60. doi: 10.1183/09031936.03.00089902. [DOI] [PubMed] [Google Scholar]
- 13.Polotsky VY, Li J, Punjabi NM, Rubin AE, Smith PL, Schwartz AR, et al. Intermittent hypoxia increases insulin resistance in genetically obese mice. J Physiol. 2003;552:253–64. doi: 10.1113/jphysiol.2003.048173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Somers VK, Dyken ME, Clary MP, Abboud FM. Sympathetic neural mechanisms in obstructive sleep apnea. J Clin Invest. 1995;96:1897–904. doi: 10.1172/JCI118235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.McArdle N, Douglas NJ. Effect of continuous positive airway pressure on sleep architecture in the sleep apnea-hypopnea syndrome: A randomized controlled trial. Am J Respir Crit Care Med. 2001;164:1459–63. doi: 10.1164/ajrccm.164.8.2008146. [DOI] [PubMed] [Google Scholar]
- 16.Saarelainen S, Lahtela J, Kallonen E. Effect of nasal CPAP treatment on insulin sensitivity and plasma leptin. J Sleep Res. 1997;6:146–7. doi: 10.1046/j.1365-2869.1997.00034.x. [DOI] [PubMed] [Google Scholar]
- 17.West SD, Nicoll DJ, Wallace TM, Matthews DR, Stradling JR. Effect of CPAP on insulin resistance and HbA1c in men with obstructive sleep apnoea and type 2 diabetes. Thorax. 2007;62:969–74. doi: 10.1136/thx.2006.074351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Barbe F, Mayoralas LR, Duran J, et al. Treatment with continuous positive airway pressure is not effective in patients with sleep apnea but no daytime sleepiness, a randomized controlled trial. Ann Intern Med. 2001;134:1015–23. doi: 10.7326/0003-4819-134-11-200106050-00007. [DOI] [PubMed] [Google Scholar]
- 19.Brooks B, Cistulli PA, Borkman M, Ross G, McGhee S, Grunstein RR, et al. Obstructive sleep apnea in obese noninsulin-dependent diabetic patients: Effect of continuous positive airway pressure treatment on insulin responsiveness. J Clin Endocrinol Metab. 1994;79:1681–5. doi: 10.1210/jcem.79.6.7989475. [DOI] [PubMed] [Google Scholar]
- 20.Smurra M, Philip P, Taillard J, Guilleminault C, Bioulac B, Gin H. CPAP treatment does not affect glucose-insulin metabolism in sleep apneic patients. Sleep Med. 2001;2:207–13. doi: 10.1016/s1389-9457(00)00079-4. [DOI] [PubMed] [Google Scholar]
- 21.Koskenvuo M, Kaprio J, Heikkilä K, Sarna S, Telakivi T, Partinen M. Snoring as a risk factor for ischaemic heart disease and stroke in men. Br Med J. 1987;294:643. doi: 10.1136/bmj.294.6572.643-c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Weyer C, Bogardus C, Mott DM, Pratley RE. The natural history of insulin secretory dysfunction and insulin resistance in the pathogenesis of type 2 diabetes mellitus. J Clin Invest. 1999;104:787–94. doi: 10.1172/JCI7231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ruige JB, Assendelft WJ, Dekker JM, Kostense PJ, Heine RJ, Bouter LM. Insulin and risk of cardiovascular disease: A meta-analysis. Circulation. 1998;97:996–1001. doi: 10.1161/01.cir.97.10.996. [DOI] [PubMed] [Google Scholar]
- 24.Barceló A, Barbé F, de la Peña M, Martinez P, Soriano JB, Piérola J, et al. Insulin resistance and daytime sleepiness in patients with sleep apnoea. Thorax. 2008;63:946–50. doi: 10.1136/thx.2007.093740. [DOI] [PubMed] [Google Scholar]
- 25.DeFronzo RA, Tobin JD, Andres R. Glucose clamp technique: A method for quantifying insulin secretion and resistance. Am J Physiol. 1979;237:E214–23. doi: 10.1152/ajpendo.1979.237.3.E214. [DOI] [PubMed] [Google Scholar]
- 26.Matthews DR, Hosker JP, Rudenski AS, Naylor BA, Treacher DF, Turner RC. Homeostasis model assessment: Insulin resistance and beta-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia. 1985;28:412–9. doi: 10.1007/BF00280883. [DOI] [PubMed] [Google Scholar]
- 27.Bonora E, Targher G, Alberiche M, Bonadonna RC, Saggiani F, Zenere MB, et al. Homeostasis model assessment closely mirrors the glucose clamp technique in the assessment of insulin sensitivity: Studies in subjects with various degrees of glucose tolerance and insulin sensitivity. Diabetes Care. 2000;23:57–63. doi: 10.2337/diacare.23.1.57. [DOI] [PubMed] [Google Scholar]
- 28.Galvin P, Ward G, Walters J, Pestell R, Koschmann M, Vaag A, et al. A simple method for quantitation of insulin sensitivity and insulin release from an intravenous glucose tolerance test. Diabet Med. 1992;9:921–8. doi: 10.1111/j.1464-5491.1992.tb01732.x. [DOI] [PubMed] [Google Scholar]
- 29.Lansang MC, Williams GH, Carroll JS. Correlation between the glucose clamp technique and the homeostasis model assessment in hypertension. Am J Hypertens. 2001;14:51–3. doi: 10.1016/s0895-7061(00)01229-2. [DOI] [PubMed] [Google Scholar]


