Abstract
Utilizing a model of post-pneumonectomy (PNY) compensatory lung growth in mice, we previously observed an increase in numbers of a putative endogenous distal airway progenitor cell population (CCSPpos/pro-SPCpos cells located at bronchoalveolar duct junctions (BADJ)), at 3, 7, and 14 days after pneumonectomy, returning to baseline at 28 days post-PNY. As the origin of these cells is poorly understood, we evaluated whether bone marrow cells contributed to the pool of these or other cells during prolonged post-PNY lung regrowth. Naïve and sex-mismatched chimeric mice underwent left PNY and were evaluated at 1, 2 and 3 months for numbers of BADJ CCSPpos/pro-SPCpos cells and presence of donor-derived marrow cells engrafted as airway or alveolar epithelium. Non-chimeric mice were also examined at 12 months after PNY for numbers of BADJ CCSPpos/pro-SPCpos cells. Notably, the right accessory lobe (RAL) continued to grow disproportionately over 12 months, a novel finding not previously described. Assessment of lung mechanics demonstrated an increase in lung stiffness following PNY, which significantly diminished over one year, but remained elevated relative to 1 year-old naïve controls. However, the number of CCSPpos/pro-SPCpos BADJ cells ≥1 month following PNY was equivalent to that found in naïve controls even after 12 months of continued RAL growth. Notably, no donor bone marrow-derived cells engrafted as airway or alveolar epithelial cells, including those at the BADJ, up to 3 months after PNY. These studies suggest that lung epithelial cells, including CCSPpos/pro-SPCpos cells, are not replenished from marrow-derived cells during post-PNY lung growth in mice.
Keywords: Lung regeneration, progenitor cell, post-pneumonectomy lung growth, bronchioalveolar stem cell
Introduction
The role of endogenous lung progenitor cells in post-natal lung regeneration or repair from injury remains unclear. Several populations of putative endogenous progenitor cells have been identified in mouse models of lung injury or regeneration. These include basal cells in the trachea and upper airways and also in the tracheal submucosal glands (1-8). In the distal airways, a number of putative progenitor populations have been proposed including toxin-resistant variant Clara cells, CD45negCD31negEpCAMhiCD49fposCD104posCD24low cells, integrin α6β4posSPCnegcells, CK5pos p63 pos cells, and a population of CCSPpos/pro-SPCpos cells located at the bronchoalveolar duct junction (BADJ) (9-13). Whether these distal airway progenitors represent similar cells differentially characterized in separate laboratories is currently the subject of intense investigation (14-17). Notably, several of these populations, including the dual-labeled BADJ CCSPpos/pro-SPCpos cells, exhibit marked proliferation when the lungs are treated with naphthalene or bleomycin, suggesting a role for these cells in epithelial repair from toxic injury (9,11,15). In addition to local sources, an extra-pulmonary origin of lung cells has been postulated to play a role in lung repair or regeneration based on reports showing rare engraftment of bone marrow derived cells as airway and/or alveolar epithelium in different models of lung injury (reviewed in 16,17). While this remains a controversial field (18-20), bone marrow cell populations that have been postulated to contribute to lung epithelial regeneration include both mesenchymal stromal cells and also rare populations of epithelial stem-progenitor cells (21-27). While current paradigms suggests a limited, if any role, of extrapulmonary cells in structural lung epithelial repair, one intriguing question that remains is whether extrapulmonary cells, notably bone marrow-derived cells can contribute to the pool of endogenous distal airway lung progenitor cells during lung regeneration, particularly in a non-injurious model of post-natal lung growth.
To further understand the potential relevance of the BADJ CCSPpos/pro-SPCpos cellsin airway or alveolar regeneration, and the potential contribution of extra-pulmonary cells to the pool of these cells, exploration of their behavior in models of lung regeneration is important. Post-pneumonectomy compensatory lung growth in adult mice is a robust model of non-injurious post-natal lung growth in which lung weight, volume, cellularity, DNA, and protein content are restored within two weeks after unilateral pneumonectomy (28-37). We recently found that numbers of BADJ CCSPpos / pro-SPCpos cells increased within three days, peaked at 220% of control cell densities at 14 days and returned to control levels at 28 days after unilateral pneumonectomy in mice (38), suggesting a potential coordination between progenitor cell proliferation and post-pneumonectomy lung regrowth. In the current study, we found that continued compensatory lung growth occurred over one year after pneumonectomy with disproportionate growth of one lobe, the right accessory lobe. We therefore followed mice for up to one year after unilateral pneumonectomy and assessed structural lung changes and lung mechanics as well as changes in the number of BADJ CCSPpos / pro-SPCpos cells and the potential contribution of bone marrow-cells in lung regrowth.
Materials and Methods
All studies were approved by the UVM IACUC and conformed to all institutional and AAALAC standards.
Bone Marrow Harvest, Culture, and Transplantation
Total marrow was obtained from the long bones of adult (8-12 weeks) male C57BL/6 mice as previously described (19,22). Adult (8 weeks) female C57BL/6 mice underwent total body irradiation (1,000 rads) using a cesium-137 cell irradiator followed by tail vein administration of total bone marrow cells (2 × 106 cells/mouse). Engraftment in our laboratory using this approach has typically been between 80-90% (19,22).
Pneumonectomy
Pneumonectomy (PNY) was performed one month after engraftment or in comparably aged naïve mice using standard surgical procedures (38,39). All procedures were performed under sterile conditions in a laminar hood in a designated procedure room in UVM’s animal facility. Mice were placed into an induction chamber with 5% isoflurane vapor until each breath was approximately 5 seconds apart and then intubated under direct laryngoscopy with a Hallowell mouse ET tube (1.22mm) using a guide wire. Mice were then ventilated with a rodent mini-ventilator (Hugo Sachs Electronick, Germany) supplemented with 2% isoflurane and oxygen. Paralube® was applied to each eye, mice placed into right lateral decubitus position (left side up), and the left side of the body shaved and sterilized with 70% ethanol and betadine. A 2cm ventral-dorsal subcutaneous incision was made followed by cutting through the muscle layer to expose the ribcage. A 1cm thoracotomy incision was then made between the 3rd and 4th rib and a retractor was then inserted to spread the rib cage and expose the lungs. Using blunt ring forceps the left lobe of the lung was pulled out of the chest cavity and piece of chromic gut 2-0 suture looped around the hilum of the lung and sutured tightly. The rest of the lung tissue was then removed and the left hilar stump was sealed with veterinary glue. Air leak was assessed by dropping sterile saline into chest cavity and observing for air bubbles. Once no air leak was observed, the surgical incisions were closed using a 6-0 chromic gut suture on the ribs, muscle and skin. Anesthesia was slowly reduced and turned off by the time the last stitch was applied. Antibiotic ointment was applied to the incision site. Mice were removed from ventilation and extubated once spontaneous breaths were initiated. Following surgery, mice received buprenorphine (0.05 mg/kg subcutaneously q 12 hr) for pain over the first 48 hours. Mice with significant post-operative distress, as assessed by movement, breathing and feeding pattern, were euthanized with high dose pentobarbital and cervical spine dislocation (38)
Assessment of Lung Mechanics
Mice were anesthetized with intraperitoneal (IP) pentobarbital (90 mg/kg), and connected to a FlexiVent mechanical ventilator (SCIREQ, Montreal) via surgical tracheostomy with an 18 gauge metal cannula. Mice received a tidal volume of 10 ml/kg at 180 breaths/minute with positive end-expiratory pressure (PEEP) of 3 cmH2O. The mice were paralyzed with IP pancuronium bromide (0.5 mg/kg), and heart rate was monitored by electrocardiogram to ensure deep anesthesia. Following a 3 min stabilization period, the level of PEEP was set at 6 cmH2O, and two 1.0 ml, volume targeted deep inflations (DI) were delivered over 4 seconds (constant flow, pressure limit of 30 cmH2O), followed by a return to quasi-sinusoidal ventilation at 180 breaths/minute. Respiratory impedance (Zrs) was determined via Fourier transform from the signals of ventilator piston volume displacement and airway opening pressure (Pao), measured during 2-second oscillatory volume perturbations, delivered immediately after, then subsequently every 20 seconds for 8 minutes. The perturbations were composed of 13 superimposed sine waves with frequencies ranging between 1 and 20.5 Hz and mutually primed to reduce harmonic distortion (40-42). Zrs was fit to the constant phase model of the viscoelastic lung, and the parameters RN and H were derived. The parameters RN and H respectively characterize the Newtonian airways resistance and elastance, or stiffness, of the lung tissues (40-42). This same protocol was then repeated at PEEPs of 3 and 1 cmH2O. Previous work has shown that the order of PEEP does not impact mechanics measurements when one standardizes volume history with deep inflations prior to measurement (41,42).
At the end of each Zrs measurement period, a quasi-static pressure-volume curve was obtained from resting residual lung volume by first dropping PEEP to 0 cmH2O, and then immediately delivering seven steps of inspiratory volume to a total volume of 40 ml/kg, followed by seven equal expiratory steps, pausing at each step for one second. Plateau Pao was measured during each pause and plotted against piston displacement volume, the latter corrected for gas compression.
CT Scanning
Following assessment of lung mechanics, mice received a lethal overdose of sodium pentobarbital (150 mg/kg body weight, IP injection) and were scanned with a GE Medical Systems eXplore RS80 micro-CAT scanner at 80 kVp, 450 mAs, 720 views, at a resolution of 0.047 mm per voxel side. Immediately prior to scanning, the lungs were inflated to 3 cm of PEEP with nitrogen to ensure uniform inflation between different mice (43,44). Isosurface renderings were generated as previously described (43). To measure lung volumes, the borders of the parenchymal lung tissue were manually traced on serial coronal sections obtained from the CT scans using Microview visualization software, version 1.2.0-b2 (GE HealthCare, London, ON, Canada) (43,44). Tracings excluded major airways and blood vessels and were performed by a single operator for consistency. Separate tracings were also made for the right accessory lobe in each scan. The volume in ml occupied by the entire lung was calculated using the Microview software by determining the number of voxels lying within the lung in a 3-dimensional image of the entire organ, and then multiplying this number by the pixel volume (1.038×10-7 ml). The volume of air within the lung was determined, as follows. X-ray density values for air and tissue were taken to be the median values of density histograms obtained from regions known to be pure tissue and pure air within a representative micro-CT image. We calculated the fraction of air in a voxel assuming a linear relationship between its x-ray density and its air fraction, calibrated to the x-ray densities corresponding to pure air and pure tissue. The total air volume in ml was then calculated as the sum of the air volumes in all lung voxels multipled by the voxel volume. This procedure was repeated for the right accessory lobe alone.
Assessment of CCSPpos / proSP-Cpos Cells in Recipient Lungs
Recipient mice were euthanized by lethal overdose of IP pentobarbital at the indicated times, the heart-lung bloc removed by dissection, and the lungs gravity fixed (20 cm H2O) with 4% paraformaldehyde for 1 hour at room temperature. Immunofluorescent staining (IF) was performed on formalin-fixed paraffin embedded sections (5 um). As primary antibodies, polyclonal goat antibody anti-CCSP (Santa Cruz 9973, dilution 1:200), polyclonal rabbit antibody anti-proSPC (Chemicon AB3786, dilution 1:1000), and monoclonal mouse antibody anti-BrdU (Santa Cruz, dilution 1:200) were used. Tissue sections were deparaffinized and hydrated using standard methods, and antigen retrieval was performed using a citrate buffer (pH6.0) and microwave heating (10 min). Tissues were washed (PBS with 0.1% Triton X-100) three times after antigen retrieval. Detection of CCSPpos/pro-SPCpos cells was performed as follows: when triple staining for co-localization of proSP-C, CCSP, and BrdU, donkey anti-rabbit Alexafluor 488 (green), donkey anti-goat Alexafluor 350 (blue), and donkey anti-mouse Alexafluor 594 (red); when staining for proSP-C and CCSP, donkey anti-rabbit Alexafluor 488 and donkey anti-goat Alexafluor 594, respectively. The appropriate single-antibody and secondary-only controls were performed and no dual staining or relevant background staining was observed. Between 16-30 bronchioalveolar duct junctions (BADJ) per mouse were photographed digitally (Nikon Eclipse E600, Spot cooled CCD camera and software) and the images merged in Adobe Photoshop 6.0. CCSPpos/pro-SPCpos cells were identified as those with a single nucleus (DAPI) and clear double staining for CCSP (rhodamine) and proSP-C (FITC), and were counted as number of BASC per BADJ (22). To ensure correct identification of CCSPpos/pro-SPCpos cells, deconvolution microscopy was utilized where necessary for colocalization of CCSP and pro-SPC within the cytoplasm of cells of interest (22). Type II alveolar epithelial cells (AECII) were identified as those with punctate staining with proSP-C. Total nucleated cells per HPF (i.e DAPI-stained nuclei) were counted, and the mean percentage of AECII cells/nucleated cells in a minimum of 15 hpf was obtained (19,22).
Assessment of Donor-Derived Cells in Recipient Lungs
Donor-derived cells were assessed by fluorescence in situ hybridization for Y chromosome positive cells followed by immunohistochemical characterization of epithelium and leukocytes using antibodies directed against Clara cell secretory protein (CCSP), pro surfactant protein C (proSP-C), and CD45 (19,22). Sections were systematically visualized with a Zeiss LSM 510 META confocal microscope. Cell counts of Y chromosome positive, CD45 positive cells (representing mature donor-derived leukocytes), of Y chromosome positive, CD45neg, proSPCpos and/or CCSPpos cells were done on 20 randomly selected 60X power fields per lung.
Statistical Analyses
Data are expressed as the mean ± standard error. To assess potential epithelial chimerism, Poisson analysis for rare events was applied to evaluate the distribution of rare donor-derived cells among the different experimental conditions (29,30,36). Changes in numbers of BADJ CCSPpos / proSP-Cpos cells were analyzed using a mixed effect model, admitting mouse as a random effect. The number of BADJ CCSPpos / proSP-Cpos cells was determined and the distribution tested for normality using the Shapiro Wilks test (38,46). The ratio was examined, using mixed effects modeling with inter-subject variability as a random effect, to see if there was an association between ratio and observation point (day). Mixed effects analysis under these situations is routinely used to admit the subject-specific aspect of variation into the analysis. Failure to do this leads to excessive type 1 errors (i.e. smaller than appropriate estimates of errors in the regression coefficients). To explore observation point (day) association with the BADJ CCSPpos / pro-SPCpos cell values, Cuzick’s trend test was utilized as there were discrete, as opposed to continuous values (or levels) for CCSPpos/proSP-Cposcells (38,44). A p-value of ≤0.05 was considered significant. Measurements of lung elastance (H) and airways resistance (RN), obtained at the end of each measurement period (at PEEP of 6, 3, and 1 cmH2O) were compared by 2-way ANOVA based on PEEP and experimental group (naïve versus PNY). Effects of PNY and PEEP were determined significant when p was <0.05. The values for Pao at each stepwise increment of volume on the inspiratory limb of the PV curves were compared between naïve and PNY mice by one-way ANOVA and reported as significant at a p< 0.05 (41,42). Differences between gas and total lung volumes were assessed by Students’ t-test (46).
Results
Lungs undergo continued growth up to one year following pneumonectomy with disproportionate growth of the right accessory lobe (RAL)
The pneumonectomy procedure was well tolerated with an overall survival of approximately 75%. When mortality occurred, it was generally in the peri-operative period and related to anesthesia complication or pneumothorax. Surviving mice thrived over the subsequent study intervals without exhibiting signs of respiratory distress. Serial CT scans with measurement of lung volumes and gross appearance at necropsy demonstrated a continued growth, notably of the right accessory lobe (RAL) over the 12 month duration of the study (Figures 1,2). Similar growth was observed in both chimeric and non-chimeric mice.
Figure 1.
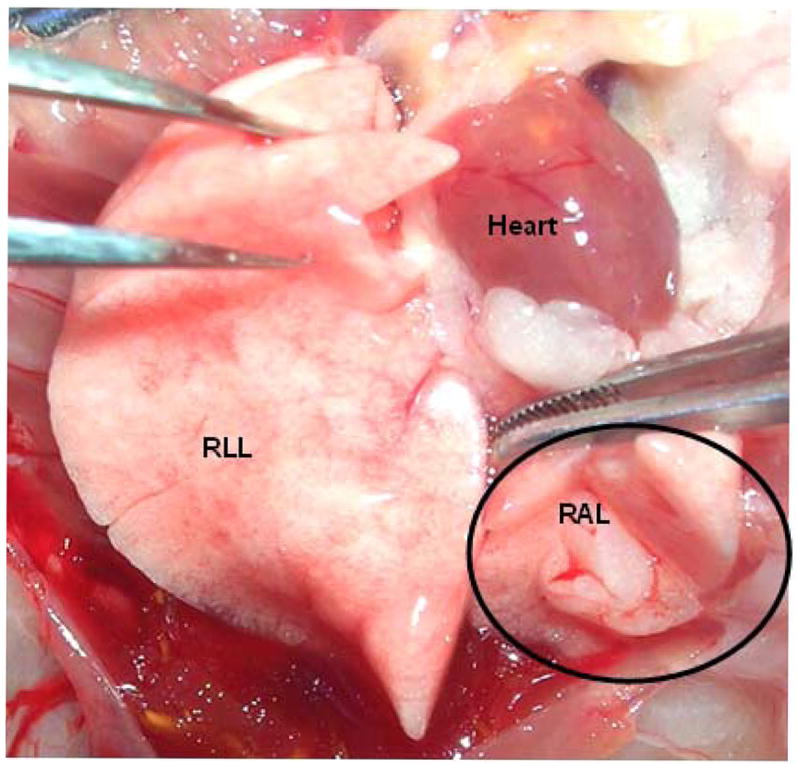
A: Representative photograph taken at necropsy 1 year after pneumonectomy demonstrates substantial growth of the right accessory lobe. RLL = right lower lobe; RAL = right accessory lobe. The circle outlines the RAL.
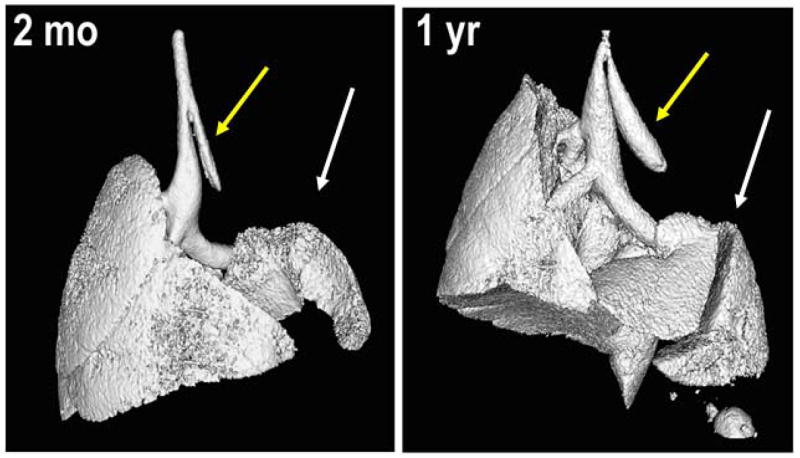
B: Representative isosurface renderings of the CT scans at 2 months and at 1 year following left pneumonectomy demonstrates growth and expansion of right lower lobe into left chest cavity (white arrows). Yellow arrows indicate the residual left main stem bronchus. Scans represent typical results obtained in 6 mice evaluated at 2 months and 7 mice evaluated 1 year after pneumonectomy.
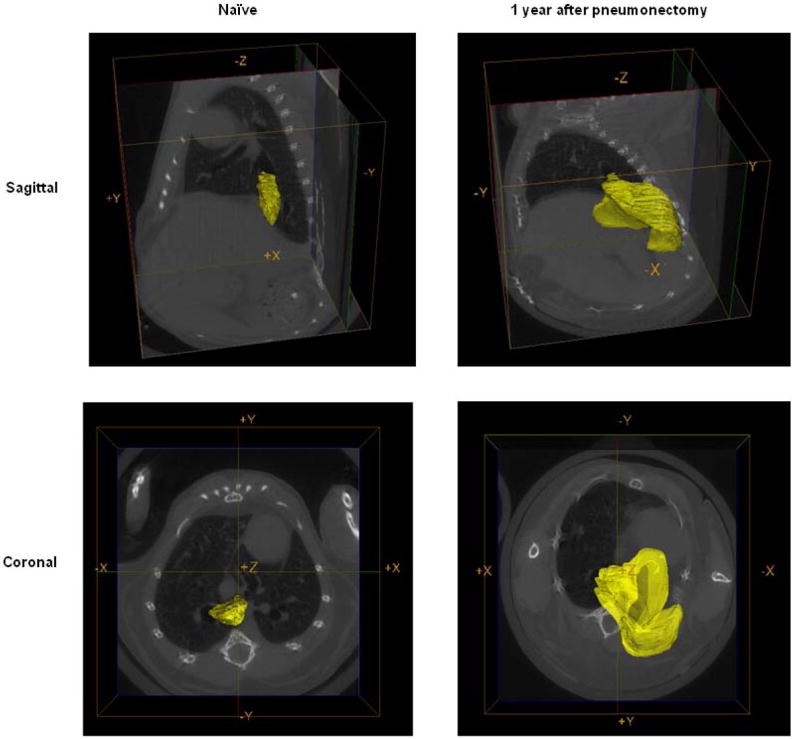
C: Representative reconstructive regions of interest on the CT scans highlighting the right accessory lobe (yellow) one year after pneumonectomy and in an age-matched naïve control mouse.
Figure 2.
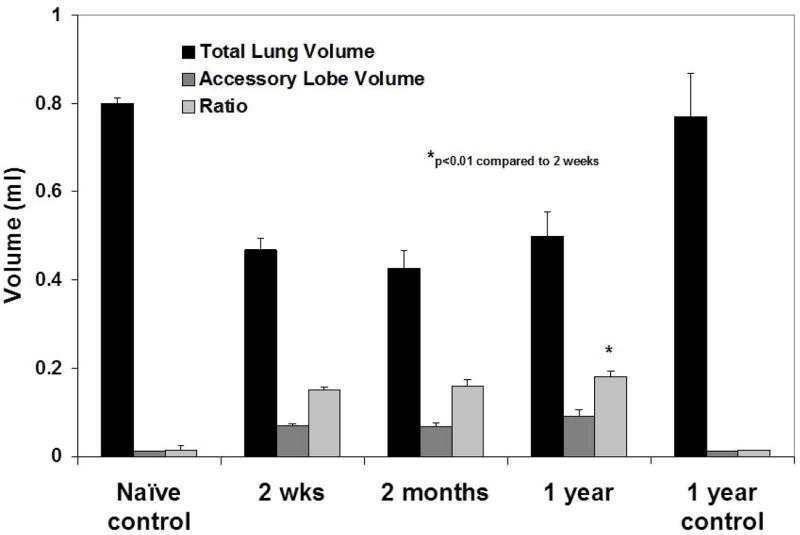
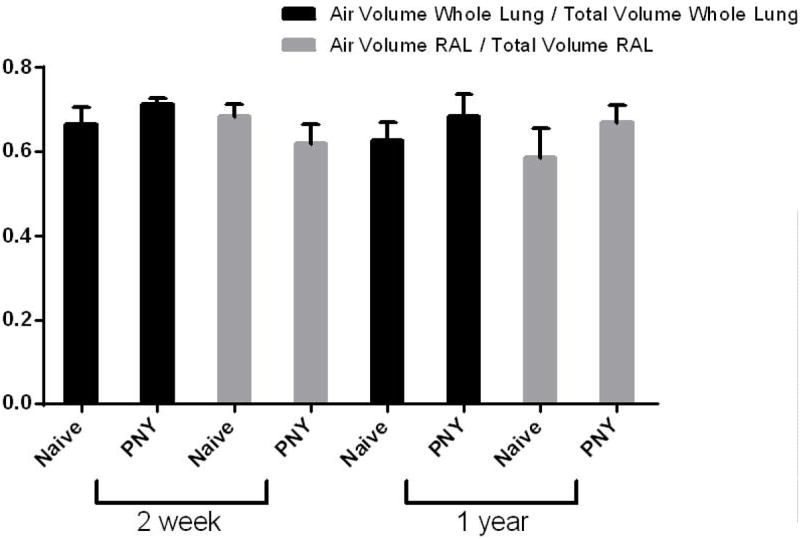
A: Quantitative assessment of the total lung volumes from the CT scans demonstrates disproportionate growth of the right accessory lobe over one year post-pneumonectomy. Values represent means ± standard deviations from 3 (naïve 2 week control, naïve 1 year control), 6 (2 month PNMO), or 7 (2 week PNMO, 1 year PNMO) representative mice assessed at each time point.
B: Assessment of air and total lung volumes demonstrates similar ratios of air/total volumes at both 2 weeks and 1 year post-pneumonectomy compared to each other and to naïve age-matched controls. Values represent means ± standard deviations from 6 (1 year naive), 7 (2 weeks PNY, 1 year PNY), or 8 (2 week naïve) mice assessed at each time point. RAL = Right accessory lobe.
To assess whether the disproportionate expansion of the RAL represented volumetric expansion resulting from disproportionate stretch or increased tissue mass, the ratios of gas volume to total lung volume were assessed at 2 weeks and at one year post-pneumonectomy and compared to naïve age-matched controls. As demonstrated in Figure 2B, similar ratios of air/total lung volumes were found in the RAL as in the whole lung at 2 weeks and 1 yr after pneumonectomy. These ratios were also similar to those observed in age-matched non-operated controls. This suggests that the RAL experienced disproportionate increases in tissue mass rather than simple volumetric expansion. There was no obvious difference in histologic appearance of the RAL compared to the other lung lobes (data not shown).
Alterations in lung mechanics persist for 1 year following pneumonectomy
Lung elastance (H) was significantly increased over the entire range of PEEP two weeks following PNY when compared to age and weight-matched naïve controls (ANOVA, <0.05) (Figure 3A). Lung elastance in naïve and PNY mice significantly declined between two weeks and one year (ANOVA, p<0.05), and the decrease in elastance was roughly equivalent in both groups except at the highest level of PEEP. However, elastance values in PNY mice never decreased to match values measured in age-matched control mice. Newtonian (airway) resistance (RN) was also significantly higher in mice that underwent PNY, both at two weeks and one year; RN also declined in both groups to the same degree between two weeks and one year after PNY (Figure 3B). The findings of increased respiratory system elastance in mice following PNY was also corroborated by the significant rightward shift of the PV curve (pressure on horizontal axis) in mice following PNY when compared to naïve control mice (ANOVA, p<0.05) both at two weeks and one year (Figure 3C).
Figure 3.
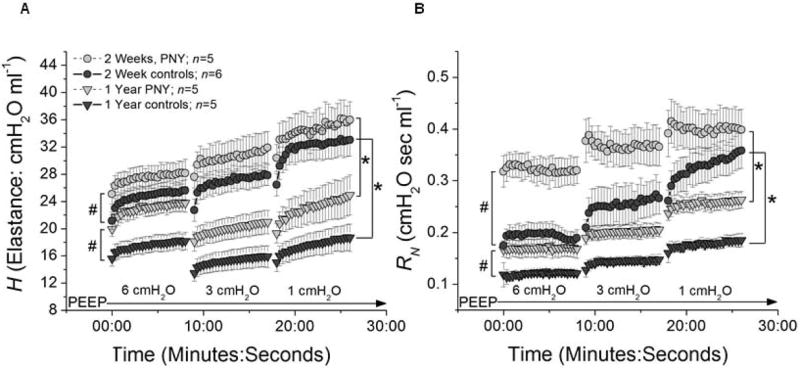
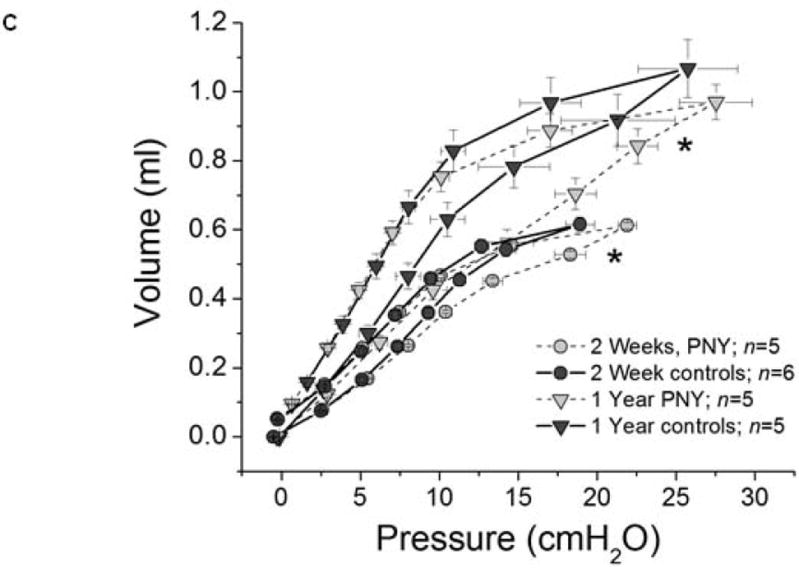
Assessment of lung mechanics demonstrates decreased stiffness over one year after pneumonectomy, consistent with continued lung growth. A) Mean values (+/- SEM) of respiratory elastance (H) plotted against time at a PEEP level of 6, 3, and 1 cm H2O for mice at two weeks after PNY (grey circles), one year after PNY (light grey triangles), and in age matched controls at 2 weeks (dark grey circles) and one year (dark grey circles). B) Mean values (+/- SEM) of airways resistance (RN) plotted against time at a PEEP level of 6, 3, and 1 cm H2O, utilizing the same key for groups as in A. For both panels, # signifies a significant difference between PNY and age-matched controls in ending values of H across all levels of PEEP (ANOVA, p<0.05). * signifies a significant difference between 2 weeks and one year in ending values of H across all levels of PEEP (ANOVA, p<0.05). C) Mean values (+/- SEM) of piston volume (ml) plotted against matching mean values of airway opening pressure (cmH2O) following 8 minutes ventilation at PEEP of 1 cm H2O for mice at two weeks after PNY (grey circles), one year after PNY (light grey triangles), and in age matched controls at 2 weeks (dark grey circles) and one year (dark grey circles). * signifies a significant difference between PNY and age-matched controls in mean airway pressure values across the entire inspiratory limb (right side) of the pressure-volume loop, a surrogate for lung stiffness (ANOVA, p<0.05). Values represent results from 3-7 representative mice assessed at each time point.
Abundance of BADJ CCSPpos / proSP-Cpos cells does not increase over one year in the expanding RAL or in any other lung lobe
To assess whether numbers of BADJ CCSPpos / proSP-Cpos cells increased over the 1 year period of continued lung growth, mounted fixed lung sections obtained from the RAL and from the other lung lobes were dual stained and the numbers of these cells counted. Representative photomicrographs at one and three months following pneumonectomy are shown in Figure 4. Quantifying the numbers of BADJ CCSPpos / proSP-Cpos cells demonstrated that, although there was a non-significant trend towards increase at 2 and 3 months, the numbers of these cells at 1, 2, 3, and 12 months following pneumonectomy did not significantly increase over levels found in naïve control lungs despite continued lung growth (Figure 5). However, there were significantly fewer BADJ CCSPpos / proSP-Cpos cells at 12 months compared to 2 months post pneumonectomy. Similar numbers of BADJ CCSPpos / proSP-Cpos cells were observed in the RAL as in the remaining right lung lobes at the different intervals post-pneumonectomy.
Figure 4.
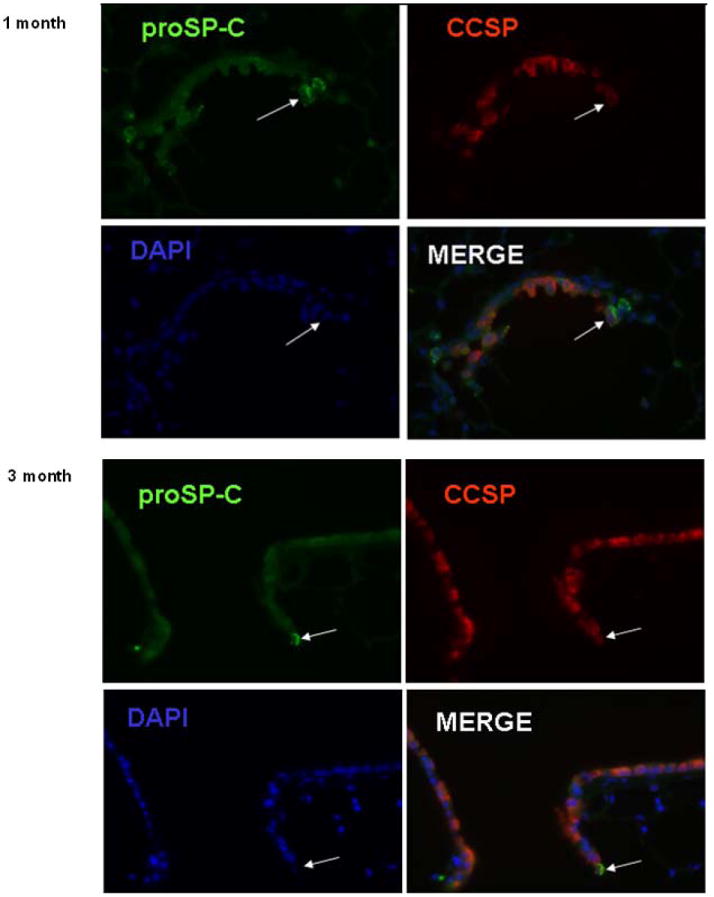
Representative photomicrographs of mouse lungs depicting dual labeled BADJ CCSPpos / pro-SPCpos cells either 1 (A) or 3 (B) months after pneumonectomy. Individual staining and the merged images are shown. Similar results were observed in mouse lungs evaluated at 2 or 12months after pneumonectomy. Values represent results from 3-6 representative mice assessed at each time point. Original Magnification 400×.
Figure 5.
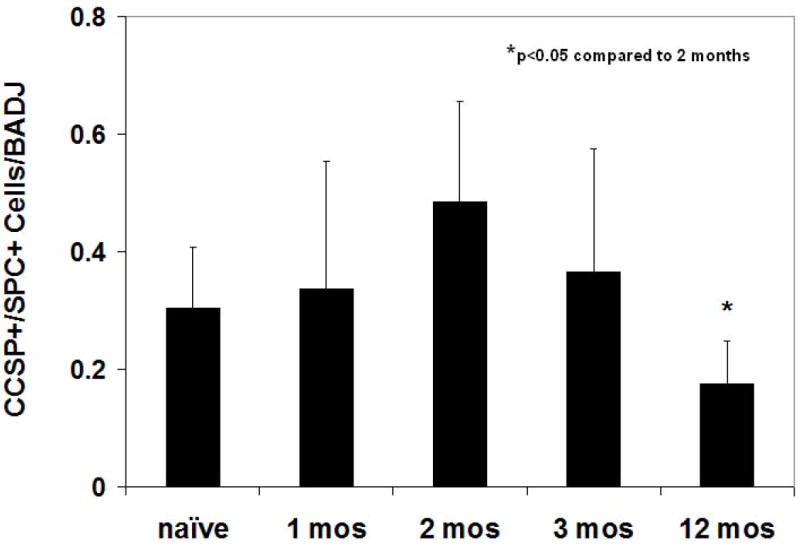
No significant increase in numbers of BADJ CCSPpos / pro-SPCpos cells over those found in naïve control lungs was observed in lungs assessed 1,2,3, or 12 months post-pneumonectomy. Bars represent means + standard deviations of 16-30 BADJ counted on each of 15-20 serial sections of each mouse lung. N = 3-7 mice for each experimental condition. *p<0.05 compared to 2 months.
No epithelial chimerism is observed up to 3 months post-pneumonectomy
Assessment of presence of donor-derived cells in recipient lungs did not demonstrate any airway epithelial chimerism derived from bone marrow up to 3 months post-pneumonectomy (Figure 6). In particular, no epithelial chimerism was observed at airway branch points or at bronchoalveolar duct junctions or alveolar septae, regions where other progenitor populations, variant toxin-resistant Clara cells and dual-labeled CCSPpos / pro-SPCpos cells, and Integrin beta4pos cells have been localized (9,11,15) (Figure 6). As we have previously found in lungs of sex-mismatched chimeric mice following myeloablation and transplantation of total marrow, rare apparent chimerism of type 2 alveolar epithelial cells was observed (data not shown, 29,30). In addition we noted abundant donor-derived CD45pos leukocytes and other inflammatory cells in the lung suggesting chimerism of the lung with hematopoietic derived cells, Other scattered rare CD45neg donor-derived cells were observed scattered throughout the lung parenchyma but were not further characterized. Quantification of airway, alveolar, and dual-labeled CCSPpos / pro-SPCpos epithelial and hematopoietic cell chimerism based on FISH is depicted in Table 1.
Figure 6.
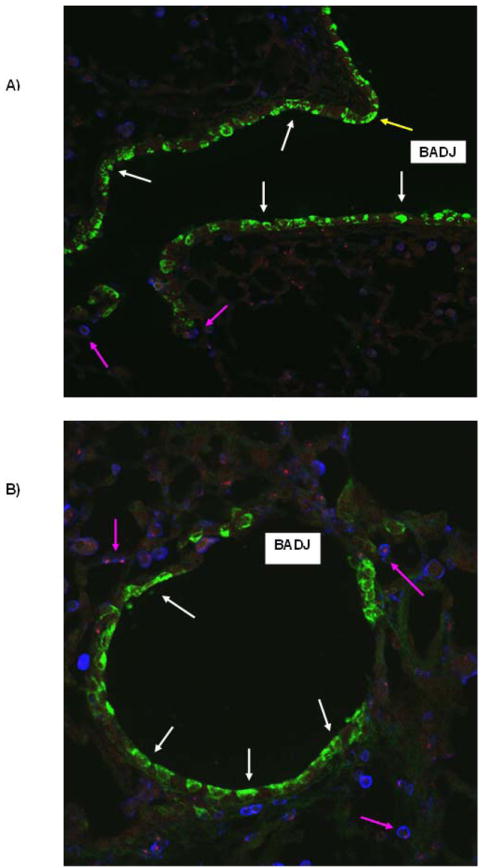
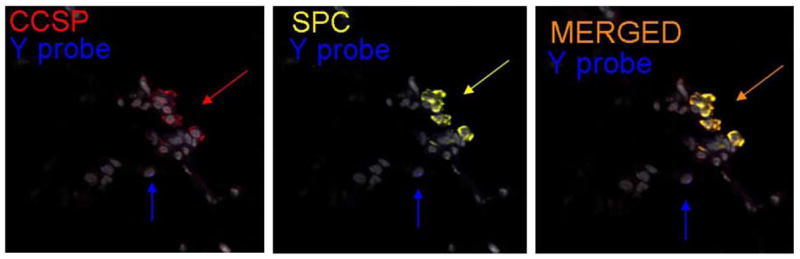
No obvious airway epithelial chimerism was observed up to 3 months after pneumonectomy in sex-mismatched chimeric mice. A) Low power view demonstrating an airway branch point (yellow arrow) and a bronchoalveolar duct junction (BADJ) (original mag 100×). B) Higher power view of a terminal bronchiole and BADJ (original mag 200×). White arrows: CCSP+ (green) airway epithelial cells; Pink arrows: CD45+ (blue) leukocytes, the majority of which were also FISH positive for the Y chromosome (red). C) Higher power view of a BADJ showing a donor derived leukocyte (FISH positive for the Y chromosome, blue, blue arrow) and individual and merged images of CCSP+ and SPC+ cells. No obvious donor-derived dual positive BADJ CCSP+ or SPC+ cells were observed (original mag 400×). Values represent results from 3-6 representative mice assessed at each time point.
Table 1.
| Experimental Group (n) | % donor-derived cells of total cells | Normalized % donor-derived cells of total cells | % CD45+ cells of Y+ cells | % pro-SPC cells of Y+ CD45 -cells | % CCSP+ cells of Y+, CD45-cells | % pro-SPC+/CCSP+ of Y+, CD45- cells |
|---|---|---|---|---|---|---|
| Pneumonectomy 1 month (n=5) | 21.2 (±3.3) | 29.0 (±4.5) | 96.3 (±3.4) | 0.2 (±0.08) | 0 | 0 |
| Pneumonectomy 2 months (n=4) | 19.4 (±2.6) | 26.6 (±3.6) | 95.8 (±4.0) | 0.1 (±0.05) | 0 | 0 |
| Pneumonectomy 3 months (n=7) | 23.2 (±2.0) | 31.8 (±2.7) | 96.2 (±3.5) | 0.2 (±0.09) | 0 | 0 |
Values represent means ±standard error
The frequency of donor-derived cells was normalized to the fraction of cells showing Y positivity by FISH in a male control (73%) to compensate for partial nuclear sampling.
Discussion
Post-pneumonectomy compensatory lung growth in adult mice is a robust model of non-injurious post-natal lung growth (28-38). While multiple lung cell types are involved, the roles of specific endogenous lung progenitor cells are not well defined. We recently found that following unilateral pneumonectomy, the numbers of one putative distal airway progenitor population, BADJ CCSPpos / pro-SPCpos cells, increased within three days, peaked at 220% of control cell densities at 14 days and returned to control levels at 28 days after unilateral pneumonectomy in mice (38). However, post-pneumonectomy lung growth is rarely assessed after the first few weeks. In the current study, we found that the lung, in particular the RAL, continued to undergo somatic growth over a one year period following left pneumonectomy. This afforded an opportunity to assess BADJ CCSPpos / pro-SPCpos cell abundance and contributions from bone marrow over a longer period of non-injurious post natal lung growth.
Consistent with previous experience with pneumonectomy in rodents (15-23) all the lung lobes increased in size over the initial post-operative period, including the right middle (cardiac) lobe, previously described to robustly increase in size in the immediate post-operative period (32,34). Also consistent with previous experience with this procedure, most compensatory growth was completed within the first several weeks after pneumonectomy (35). However, in addition to subsequent continued expansion of all lobes over the one year period of observation, the right accessory lobe (RAL) expanded disproportionally for 12 months in the non-chimeric mice where long-term measurements were made. This previously unrecognized phenomenon of disproportionate growth of the RAL appears to reflect tissue growth (maintenance of air/total tissue volume) rather than simple volumetic expansion as assessed by CT analyses over time (Figure 2). This continued lung growth was paralleled by a roughly equivalent decline in lung elastance between 2 weeks and 1 year in naïve mice and those undergoing pneumonectomy, when elastance was measured at low or moderate PEEP levels. Since the ratio of air volume to lung volume did not appreciably change between 2 weeks and 1 year of age (i.e. similar changes in lung stretch and aeration at resting lung volume), the changes in elastance between 2 weeks and one year are most likely attributable to changes in lung and thoracic volume as opposed to intrinsic changes in tissue viscoelasticity. The same holds for differences observed in the PV curves over one year, which were controlled for mouse weight by inflating lungs to a weight-matched volume (40ml/kg).
As PNY and age-matched control mice matured over one year, both groups demonstrated similar patterns of increasing airways resistance (RN) with de-escalating levels of PEEP (Figure 3B), signifying similar changes in airway-parenchymal interdependence with age. However, when examining the PEEP response in elastance (H) (Figure 3A), whereas mice at two weeks demonstrate a progressive decline in lung inflation and compliance (increasing elastance) with diminishing PEEP from 6 to 1 cm H2O, 1 year-old mice demonstrate an increase in elastance when going from PEEP of 6 to 3 cm H2O. This suggests that the older lungs reach their tissue lengthening limits and begin to over-distend at the higher limits of PEEP (43). In theory this phenomenon may be explained by a nonlinear distribution of collagen and elastin fibers (44) that changes with age. What the data did not demonstrate was any significant compensatory decrease in elastance over one year following PNY to suggest any significant compensatory change in total lung volume one year after PNY, which is in keeping with findings from CT estimates of total lung volume (Figure 2).
Despite continued lung growth, no significant increase in the cell density of BADJ CCSPpos / pro-SPCpos cells was observed at ≥ 1 month after pneumonectomy and none of the BADJ CCSPpos/pro-SPCpos cells detected in situ appeared to be of bone marrow origin. The lack of significant airway or alveolar chimerism also argues that bone marrow-derived cells did not contribute to other putative distal airway progenitor cell populations in addition to the dual-labeled CCSPpos / pro-SPCpos cells. Comparable results were observed by Voswinckel and colleagues who did not observe a contribution of bone marrow-derived to lung vascular growth 21 days after pneumonectomy in adult mice (37). In that study bone marrow derived cells which expressed LacZ under endothelial specific promoters (Tie-2 or flk-1) or eGFP under control of a ubiquitous promoter (cac) were not found to engraft the lung as endothelial cells when assessed 3 weeks after PNY (27). Apart from endothelial cells, the majority of cells derived from ubiquitous eGFP mice were found to be CD45pos and presumed to be leukocytes, including some cells with elongated morphology speculated to be dendritic cells. Nonetheless, the CD45pos and CD45neg cells demonstrated to seed the lung from the bone marrow after PNY may contribute to ongoing somatic growth or homeostasis of the lung and thus warrant further study. For example, recent data suggests that macrophages proliferate locally and express genes encoding pro-angiogenic proteins post-PNY, but their contribution to post-PNY compensatory growth or ongoing somatic growth is presently unknown (47).
The current results agree with our previous findings in which the abundance of BADJ CCSPpos/pro-SPCpos returned to pre-PNY values at 1 mo post-PNY (38), and extend those observations for several months. Although no further increase in BADJ CCSPpos/pro-SPCpos density was observed during prolonged growth after PNY we did not determine whether these cells differentiated into epithelial cells (Clara, Type I or II pneumocytes) as implied by in vitro studies by Kim and colleagues (9). Lineage tracing and transplantation studies will improve our understanding of the hierarchy of putative resident progenitor cells and more committed cells that participate in repair and regeneration of lung epithelium.
In parallel with studies of endogenous lung progenitor cells, the ability of extrapulmonary cells from other sources, notably those derived from adult bone marrow or from umbilical cord blood, to engraft as airway or alveolar epithelial cells has been the subject of intensive investigation (16-27). While initial studies were suggestive of substantive engraftment, the overall consensus at present is that airway or alveolar epithelial engraftment by marrow-derived cells is a rare occurrence of unclear physiologic significance. Nonetheless, some degree of limited engraftment of airway and/or airway alveolar epithelium by different types of bone marrow-derived cells continues to be suggested in different models of lung injury in mice (21-27). As some degree of lung injury is required to observe even rare putative engraftment, the non-injurious nature of post-pneumonectomy lung regrowth likely precludes potential engraftment, including populations of the different described putative distal airway epithelial progenitor populations.
The current results suggest that, at least in this model, that BADJ CCSPpos/proSP-Cpos cells do not continue to increase in frequency in prolonged post-pneumonectomy lung growth or become replenished by cells of marrow-origin in the short or long term after PNY; in fact these double positive cells decline with age suggestive of an age-related change in the niche that controls the survival or phenotype of these cells. This finding further diminishes the potential role of marrow-derived cells in lung repair after PNY. Nonetheless, the precise role of these and other endogenous lung stem and progenitor cells remains to be determinedand there remains much to be learned about endogenous stem and lung progenitor cells, including clarification of human counterparts to the cells identified in mouse models, particularly in clinical lung disease models.
Acknowledgments
The authors thank Jason Bates, John Thompson-Figueroa and Michael Sullivan of the Vermont Lung Center for assistance with the CT imaging and Robert Prenovitz (Vermont Lung Center), Christopher Terrien III MD, and Joseph Schmoker MD (UVM Department of Surgery) for assistance with the pneumonectomy procedures.
Research Support: T32 HL76122 (Vermont Lung Center, Charles Irvin, PI) NIH/NHBLI Multidisciplinary Training in Lung Biology (VS), HL081289 from the National Heart Lung and Blood Institute (DJW), Research Grants from the Cystic Fibrosis Foundation (DJW) and the American Lung Association (DJW), NCRR COBRE P20 RR-155557 (Vermont Lung Center, Charles Irvin, PI), the Italian Cystic Fibrosis Research Foundation (grant FFC#5/2006) with the contribution of “Calzedonia” and “Montblanc Italia” (RL), and 5R01HL072780-02 (Ed Ingenito PI)
Footnotes
Author Contributions
Phillip Eisenhauer: conception and design, collection and/or assembly of data, data analysis and interpretation, manuscript writing, final manuscript approval
Benjamin Earle: collection and/or assembly of data, data analysis and interpretation
Roberto Loi: conception and design, collection and/or assembly of data, data analysis and interpretation, manuscript writing, final manuscript approval
Viranuj Sueblinvong: conception and design, collection and/or assembly of data, data analysis and interpretation, manuscript writing, final manuscript approval
Meagan Goodwin: collection and/or assembly of data, data analysis and interpretation, manuscript writing, final manuscript approval
Gilman B. Allen: conception and design, collection and/or assembly of data, data analysis and interpretation, final manuscript approval
Lennart Lundblad: conception and design, collection and/or assembly of data, data analysis and interpretation, final manuscript approval
Melissa R. Mazan: collection and/or assembly of data, data analysis and interpretation
Andrew M. Hoffman: conception and design, financial support, collection and/or assembly of data, data analysis and interpretation, manuscript writing, final manuscript approval
Daniel J. Weiss: conception and design, financial support, collection and/or assembly of data, data analysis and interpretation, manuscript writing, final manuscript approval
References
- 1.Borthwick DW, Shahbazian M, Krantz QT, Dorin JR, Randell SH. Evidence for stem-cell niches in the tracheal epithelium. Am J Respir Cell Mol Biol. 2001;24:662–70. doi: 10.1165/ajrcmb.24.6.4217. [DOI] [PubMed] [Google Scholar]
- 2.Daniely Y, Liao G, Dixon D, Linnoila RI, Lori A, Randell SH, et al. Critical role of p63 in the development of a normal esophageal and tracheobronchial epithelium. Am J Physiol Cell Physiol. 2004;287:C171–81. doi: 10.1152/ajpcell.00226.2003. [DOI] [PubMed] [Google Scholar]
- 3.Schoch KG, Lori A, Burns KA, Eldred T, Olsen JC, Randell SH, et al. A subset of mouse tracheal epithelial basal cells generates large colonies in vitro. Am J Physiol Lung Cell Mol Physiol. 2004;286:L631–42. doi: 10.1152/ajplung.00112.2003. [DOI] [PubMed] [Google Scholar]
- 4.Rock JR, Onaitis MW, Rawlins EL, Lu Y, Clark CP, Xue Y, et al. Basal cells as stem cells of the mouse trachea and human airway epithelium. Proc Natl Acad Sci USA. 2009;106:12771–5. doi: 10.1073/pnas.0906850106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hong KU, Reynolds SD, Watkins S, Fuchs E, Stripp BR. In vivo differentiation potential of basal cells: Evidence for multipotent and unipotent subpopulations. Am J Physiol Lung Cell Mol Physiol. 2004;286:L643–9. doi: 10.1152/ajplung.00155.2003. [DOI] [PubMed] [Google Scholar]
- 6.Hong KU, Reynolds SD, Watkins S, Fuchs E, Stripp BR. Basal cells are a multipotent progenitor capable of renewing the bronchial epithelium. Am J Pathol. 2004;164:577–88. doi: 10.1016/S0002-9440(10)63147-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ghosh M, Brechbuhl HM, Smith RW, Li B, Hicks DA, Titchner T, et al. Context-dependent differentiation of multipotential keratin 14-expressing tracheal basal cells. Am J Respir Cell Mol Biol. 2011;45:403–10. doi: 10.1165/rcmb.2010-0283OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hegab AE, Ha VL, Gilbert JL, Zhang KX, Malkoski SP, Chon AT, et al. Novel Stem/Progenitor Cell Population from Murine Tracheal Submucosal Gland Ducts with Multipotent Regenerative Potential. Stem Cells. 2011;29:1283–1293. doi: 10.1002/stem.680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kim CF, Jackson EL, Woolfenden AE, Lawrence S, Babar I, Vogel S, et al. Identification of bronchioalveolar stem cells in normal lung and lung cancer. Cell. 2005;121:823–35. doi: 10.1016/j.cell.2005.03.032. [DOI] [PubMed] [Google Scholar]
- 10.McQualter JL, Yuen K, Williams B, Bertoncello I. Evidence of an epithelial stem/progenitor cell hierarchy in the adult mouse lung. Proc Natl Acad Sci U S A. 2010;107:1414–9. doi: 10.1073/pnas.0909207107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Teisanu RM, Chen H, Matsumoto K, McQualter JL, Potts E, Foster WM, et al. Functional analysis of two distinct bronchiolar progenitors during lung injury and repair. Am J Respir Cell Mol Biol. 2011;44(6):794–803. doi: 10.1165/rcmb.2010-0098OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chapman HA, Li X, Alexander JP, Brumwell A, Lorizio W, Tan K, et al. Integrin β6α4 identifies an adult distal lung epithelial population with regenerative potential in mice. J Clin Invest. 2011;121:2855–62. doi: 10.1172/JCI57673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kumar PA, Hu Y, Yamamoto Y, Hoe NB, Wei TS, Mu D, et al. Distal airway stem cells yield alveoli in vitro and during lung regeneration following H1N1 infection. Cell. 2011;147(3):525–38. doi: 10.1016/j.cell.2011.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rawlins EL, Hogan BL. Epithelial stem cells of the lung: privileged few or opportunities for many? Development. 2006;133(13):2455–65. doi: 10.1242/dev.02407. [DOI] [PubMed] [Google Scholar]
- 15.Stripp BR, Reynolds SD. Maintenance and repair of the bronchiolar epithelium. Proceedings of the American Thoracic Society. 2008;5(3):328–33. doi: 10.1513/pats.200711-167DR. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Weiss DJ, Bertoncello I, Borok Z, Kim C, Panoskaltsis-Mortari A, Reynolds S, Rojas M, Stripp B, Warburton D, Prockop DJ. Stem cells and cell therapies in lung biology and lung diseases. Proceedings of the American Thoracic Society. 2011;8(3):223–72. doi: 10.1513/pats.201012-071DW. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lau AN, Goodwin M, Kim CF, Weiss DJ. Stem Cells and Regenerative Medicine in Lung Biology and Diseases. Mol Ther. 2012;20(6):1116–30. doi: 10.1038/mt.2012.37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Herzog EL, Van Arnam J, Hu B, Krause DS. Threshold of lung injury required for the appearance of marrow-derived lung epithelia. Stem Cells. 2006;24(8):1986–92. doi: 10.1634/stemcells.2005-0579. [DOI] [PubMed] [Google Scholar]
- 19.Beckett T, Loi R, Prenovitz R, Poynter M, Goncz KK, Suratt BT, Weiss DJ. Acute lung injury with endotoxin or NO2 does not enhance development of airway epithelium from bone marrow. Mol Ther. 2005;12(4):680–6. doi: 10.1016/j.ymthe.2005.05.007. [DOI] [PubMed] [Google Scholar]
- 20.Kotton DN, Fabian AJ, Mulligan RC. Failure of bone marrow to reconstitute lung epithelium. Am J Respir Cell Mol Biol. 2005;33(4):328–34. doi: 10.1165/rcmb.2005-0175RC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gomperts BN, Belperio JA, Rao PN, Randell SH, Fishbein MC, Burdick MD, Strieter RM. Circulating progenitor epithelial cells traffic via CXCR4/CXCL12 in response to airway injury. J Immunol. 2006;176(3):1916–27. doi: 10.4049/jimmunol.176.3.1916. [DOI] [PubMed] [Google Scholar]
- 22.Loi R, Beckett T, Goncz KK, Suratt BT, Weiss DJ. Limited restoration of cystic fibrosis lung epithelium in vivo with adult marrow derived cells. Am J Resp Crit Care Med. 2006;173:171–179. doi: 10.1164/rccm.200502-309OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wong AP, Dutly AE, Sacher A, Lee H, Hwang DM, Liu M, Keshavjee S, Hu J, Waddell TK. Targeted cell replacement with bone marrow cells for airway epithelial regeneration. Am J Physiol Lung Cell Mol Physiol. 2007;293(3):L740–52. doi: 10.1152/ajplung.00050.2007. [DOI] [PubMed] [Google Scholar]
- 24.Wong AP, Keating A, Lu WY, Duchesneau P, Wang X, Sacher A, Hu J, Waddell TK. Identification of a bone marrow-derived epithelial-like population capable of repopulating injured mouse airway epithelium. Journal of Clinical Investigation. 2009;119(2):336–48. doi: 10.1172/JCI36882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Duchesneau P, Wong AP, Waddell TK. Optimization of targeted cell replacement therapy: a new approach for lung disease. Molecular Therapy: the Journal of the American Society of Gene Therapy. 2010;18(10):1830–6. doi: 10.1038/mt.2010.142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Yan C, Qu P, Du H. Myeloid-specific expression of Stat3C results in conversion of bone marrow mesenchymal stem cells into alveolar type II epithelial cells in the lung. Science China Life Sci. 2012;55(7):576–90. doi: 10.1007/s11427-012-4339-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kassmer SH, Bruscia EM, Zhang PX, Krause DS. Nonhematopoietic cells are the primary source of bone marrow-derived lung epithelial cells. Stem Cells. 2012;30(3):491–9. doi: 10.1002/stem.1003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Brown LM, Rannels SR, Rannels DE. Implications of post-pneumonectomy compensatory lung growth in pulmonary physiology and disease. Respir Res. 2001;2(6):340–7. doi: 10.1186/rr84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Brody JS, Burki R, Kaplan N. Deoxyribonucleic acid synthesis in lung cells during compensatory lung growth after pneumonectomy. Am Rev Respir Dis. 1978;117(2):307–16. doi: 10.1164/arrd.1978.117.2.307. [DOI] [PubMed] [Google Scholar]
- 30.Brody JS, et al. Time course of and stimuli to compensatory growth of the lung after pneumonectomy. J Clin Invest. 1975;56(4):897–904. doi: 10.1172/JCI108169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Rannels DE, et al. Cellular changes in the lungs of adrenalectomized rats following left pneumonectomy. Am J Respir Cell Mol Biol. 1991;5(4):351–62. doi: 10.1165/ajrcmb/5.4.351. [DOI] [PubMed] [Google Scholar]
- 32.Rannels DE, et al. Role of physical forces in compensatory growth of the lung. Am J Physiol. 1989;257(4 Pt 1):L179–89. doi: 10.1152/ajplung.1989.257.4.L179. [DOI] [PubMed] [Google Scholar]
- 33.Uhal BD, Hess GD, Rannels DE. Density-independent isolation of type II pneumocytes after partial pneumonectomy. Am J Physiol. 1989;256(3 Pt 1):C515–21. doi: 10.1152/ajpcell.1989.256.3.C515. [DOI] [PubMed] [Google Scholar]
- 34.Rannels DE, Rannels SR. Compensatory growth of the lung following partial pneumonectomy. Exp Lung Res. 1988;14(2):157–82. doi: 10.3109/01902148809115122. [DOI] [PubMed] [Google Scholar]
- 35.Voswinckel R, et al. Characterisation of post-pneumonectomy lung growth in adult mice. Eur Respir J. 2004;24(4):524–32. doi: 10.1183/09031936.04.10004904. [DOI] [PubMed] [Google Scholar]
- 36.Kuboi S, et al. DNA synthesis and related enzymes altered in compensatory lung growth in rats. Scand J Clin Lab Invest. 1992;52(7):707–15. doi: 10.3109/00365519209115516. [DOI] [PubMed] [Google Scholar]
- 37.Voswinckel R, Ziegelhoeffer T, Heil M, Kostin S, Breier G, Mehling T, Haberberger R, Clauss M, Gaumann A, Schaper W, Seeger W. Circulating vascular progenitor cells do not contribute to compensatory lung growth. Circulation Research. 2003;93(4):372–9. doi: 10.1161/01.RES.0000087643.60150.C2. [DOI] [PubMed] [Google Scholar]
- 38.Nolen-Walston RD, Kim CF, Mazan MR, Ingenito EP, Gruntman AM, Tsai, Boston R, Woolfenden AE, Jacks T, Hoffman AM. Cellular kinetics and modeling of bronchioalveolar stem cell response during lung regeneration. Am J Physiol Lung Cell Mol Physiol. 2008;294:L1158–L1165. doi: 10.1152/ajplung.00298.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Sakurai MK, Greene AK, Wilson J, Fauza D, Puder M. Pneumonectomy in the mouse: technique and perioperative management. Journal of Investigative Surgery. 2005;18(4):201–5. doi: 10.1080/08941930591004485. [DOI] [PubMed] [Google Scholar]
- 40.Gomes RF, Shardonofsky F, Eidelman DH, Bates JHT. Respiratory mechanics and lung development in the rat from early age to adulthood. J Appl Physiol. 2001;90(5):1631–8. doi: 10.1152/jappl.2001.90.5.1631. [DOI] [PubMed] [Google Scholar]
- 41.Allen GB, Suratt BT, Rinaldi L, Petty JM, Bates JH. Choosing the frequency of deep inflation in mice: balancing recruitment against ventilator-induced lung injury. Am J Physiol Lung Cell Mol Physiol. 2006;291:L710–717. doi: 10.1152/ajplung.00532.2005. [DOI] [PubMed] [Google Scholar]
- 42.Allen GB, Pavone LA, DiRocco JD, Bates JHT, Nieman GF. Pulmonary Impedance and Alveolar Instability During Injurious Ventilation in Rats. J Appl Physiol. 2005;99:723–730. doi: 10.1152/japplphysiol.01339.2004. [DOI] [PubMed] [Google Scholar]
- 43.Lundblad LK, Thompson-Figueroa J, Leclair T, Sullivan MJ, Poynter ME, Irvin CG, Bates JH. Tumor necrosis factor-alpha overexpression in lung disease: a single cause behind a complex phenotype. Am J Respir Crit Care Med. 2005;171(12):1363–70. doi: 10.1164/rccm.200410-1349OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Maksym GN, Bates JHT. A distributed nonlinear model of lung tissue elasticity. J Appl Physiol. 1997;82:32–41. doi: 10.1152/jappl.1997.82.1.32. [DOI] [PubMed] [Google Scholar]
- 45.Lundblad LK, Thompson-Figueroa J, Allen GB, Rinaldi L, Norton RJ, Irvin CG, Bates JH. Airway hyperresponsiveness in allergically inflamed mice: the role of airway closure. American Journal of Respiratory & Critical Care Medicine. 2007;175(8):768–74. doi: 10.1164/rccm.200610-1410OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Zar J. Biostatistical Analysis. N.J.: Prentice-Hall, Inc; 1974. [Google Scholar]
- 47.Chamoto K, Gibney BC, Ackermann M, Lee GS, Lin M, Konerding MA, Tsuda A, Mentzer SJ. Albeolar macrophage dynamics in murine lung regeneration. J Cell Physiol. 2012 Sep;227(9):3208–15. doi: 10.1002/jcp.24009. [DOI] [PMC free article] [PubMed] [Google Scholar]


