Figure 6. Free energies for water and KCl transport by KCC2 at different values of [Cl−]i.
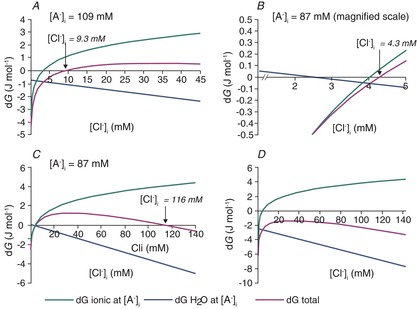
A, conditions are as described for Fig. 5. At the value of [A−]i = 109 mm, the osmotic gradient is zero in Fig. 5A. The free energy of KCl cotransport, the free energy of water movement across the membrane and the sum of these free energies [eqns (12) and (15)] are shown for different values of [Cl−]i. The sum of the ionic and water-movement free energies is zero for [Cl−]i = 9.3 mm; this is the equilibrium point for the cotransport of potassium, chloride and water via KCC2. B, the same relationships are shown for a different value of [A−]i, 87 mm, to illustrate that the free energy for ionic transport is zero at a different value of [Cl−]i from the free energy minimum for water transport or the minimum of the sum of the free energies of ion and water transport. In this example, the equilibrium value of [Cl−]i is 4.3 mm. C, a second free energy minimum exists for the parameters shown in B, at a [Cl−]i of 116 mm. The high-[Cl−]i solutions are not illustrated in Fig. 5 because they are not observed experimentally and because the corresponding transmembrane osmotic gradient is so high (in this case, 167 mosmol l−1). D, for many values of intra- and extracellular diffusible water, eqn (15) cannot be satisfied. In this case, the sum of ionic and free water transport is never zero because the gradient for water diffusion is too large. The difference in intra- vs. extracellular diffusible water was set to 0.36% in this example; other parameters are the same as for Fig. 5.
