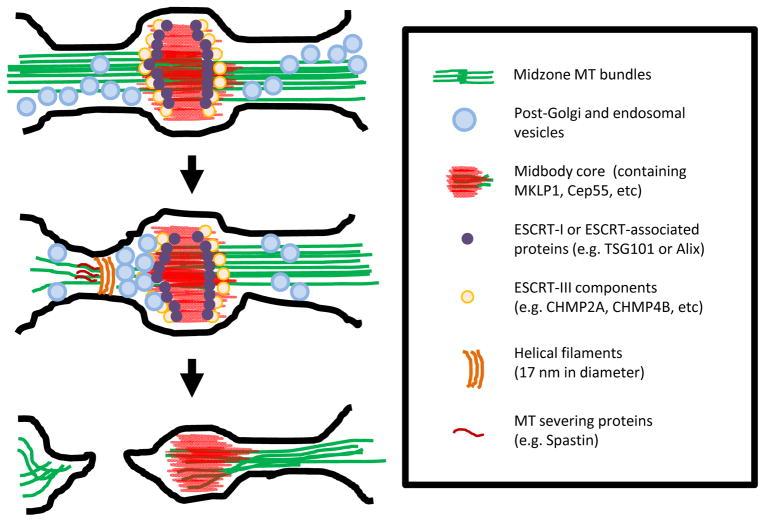
Fig 2. Multiple pathways are required for accomplishing abscission.
Multiple pathways that contribute to abscission are represented schematically from top to bottom. Top: As the cell approaches abscission, ESCRT-I (purple ovals), ESCRT-associated protein (purple ovals) and later ESCRT-III (pale yellow ovals) are recruited to the midbody (red). Meanwhile, the intercellular bridge continues to remodel, the bridge microtubules (green) gradually compact, and different types of vesicles (light blue ovals) selectively traffic to the midbody. Middle: About 10–20 minutes before abscission, vesicles are increasingly recruited, presumably facilitating remodeling of the bridge membrane in vicinity of the midbody. Concordantly, 17-nm helical filaments (dark brown stripes) whose presence depends on ESCRT-III components appear at the ultimate bridge scission site; these filaments may be spirally grown from the midbody or directly formed at this site. Spastin (dark red stripes) is also concentrated at the same site to selectively sever microtubules. These aforementioned events appear to all occur 10–20 minutes prior to the final physical separation of daughter cells, suggesting that they either orchestrate abscission or set up stage for further downstream events. Bottom: After the first bridge-severing event, abscission is accomplished and the two daughter cells are physically separated but some cells have been documented to have a second severing event.