Table 3. Inhibitory Potencies of Carboxamides 5f–5v Obtained Using an in Vitro IN Assaya.
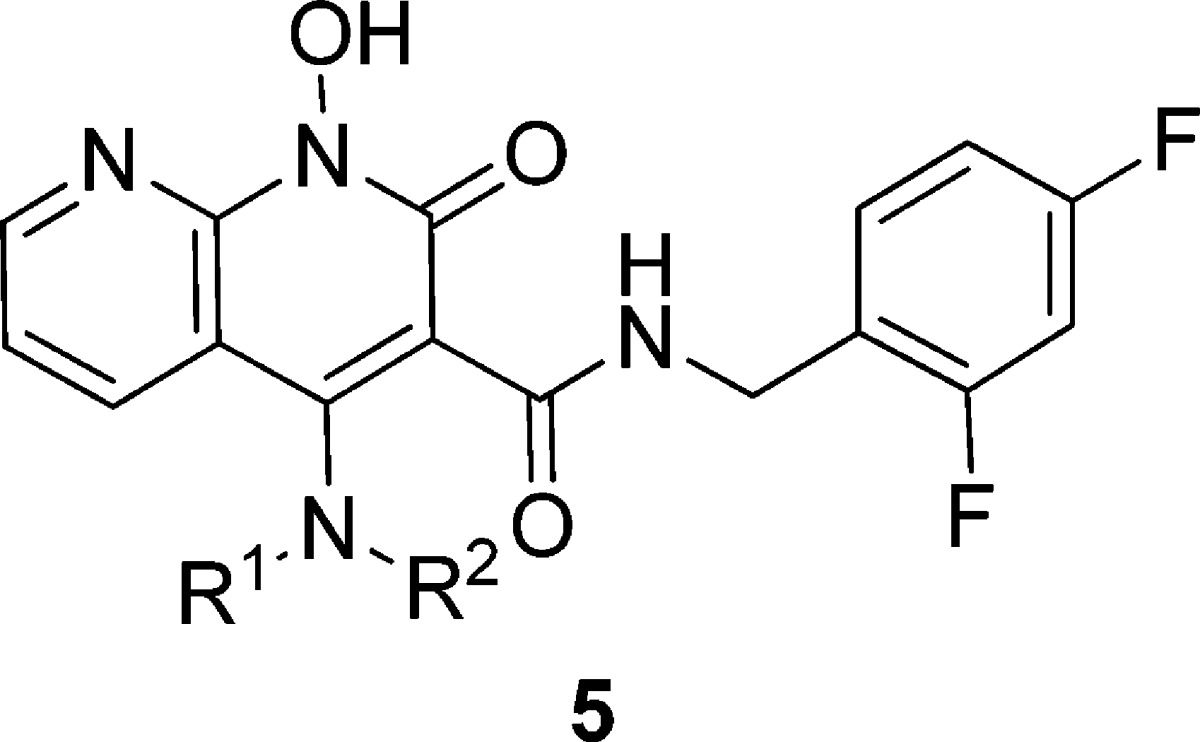
| IC50 (μM) |
||||
|---|---|---|---|---|
| compd | R1 | R2 | 3′-processing | strand transfer |
| 5f | –CH3 | –H | 3.7 ± 0.4 | 0.027 ± 0.004 |
| 5g | –CH3 | –CH3 | 21 ± 2 | 0.087 ± 0.012 |
| 5h | –morpholino | 77 ± 12 | 0.079 ± 0.013 | |
| 5i | –cycloheptyl | –H | 13 ± 1.1 | 0.46 ± 0.18 |
| 5j | –CH2CH2Ph | –H | 8.0 ± 1.5 | 0.050 ± 0.012 |
| 5k | –CH2(CH2)3Ph | –H | 12 ± 2.0 | 0.28 ± 0.11 |
| 5l | –CH2(CH2)3CH3 | –H | 8.2 ± 1.6 | 0.024 ± 0.009 |
| 5m | –CH(CH3)2 | –H | 1.8 ± 0.2 | 0.016 ± 0.004 |
| 5n | –CH2CH2NH2 | –H | 4.5 ± 0.2 | 0.039 ± 0.006 |
| 5o | –CH2CH2OH | –H | 0.55 ± 0.07 | 0.010 ± 0.009 |
| 5p | –CH2CH2OAc | –H | 5.3 ± 0.5 | 0.027 ± 0.006 |
| 5q | –CH2CO2CH3 | –H | 0.71 ± 0.10 | 0.021 ± 0.011 |
| (S)-5r | –NHCH(CH3)CO2CH3 | –H | 7.4 ± 0.8 | 0.017 ± 0.011 |
| (R)-5r | –NHCH(CH3)CO2CH3 | –H | 5.8 ± 0.6 | 0.027 ± 0.005 |
| (S)-5s | –NHCH(Ph)CO2CH3 | –H | 16.7 ± 1.4 | 0.010 ± 0.002 |
| (R)-5s | –NHCH(Ph)CO2CH3 | –H | 13.5 ± 1.0 | 0.0082 ± 0.0015 |
| (S)-5t | –NHCH(CH2OH)CO2CH3 | –H | 5.8 ± 0.5 | 0.0084 ± 0.0032 |
| (R)-5t | –NHCH(CH2OH)CO2CH3 | –H | 4.4 ± 0.5 | 0.013 ± 0.04 |
| (S)-5u | –Pro-OEt | 86 ± 6 | 0.31 ± 0.04 | |
| 5v | –H | –H | 2.5 ± 0.3 | 0.019 ± 0.002 |
