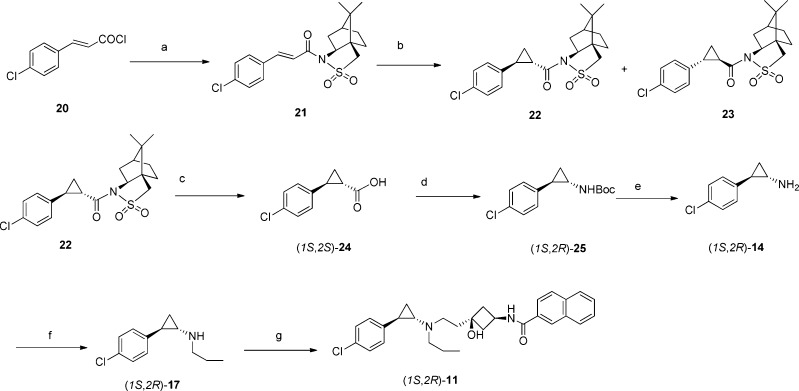
Scheme 2. Synthesis of (1S,2R)-11.
Reagents and conditions: (a) (1R)-(+)-2,10-camphorsultam, NaH, THF, 0 °C, 30 min, then RT overnight; (b) CH2N2, Pd(OAc)2, DCM, RT, 10 h; (c) (i)Ti(iOPr)4, BzOH, 150 °C, 30 min, (ii) 2 M LiOH, MeOH, RT, 2 h, (iii) 4 M HCI; (d) (i) ethyl chloroformate. Et3N, acetone, 0 °C, 2 h, (ii) NaN3,1 h, (iii) 90 °C, toluene, 3 h, (iv) ButOH, reflux, 16 h. (e) TFA, DCM, RT, 12 h; (f) propionaldehyde, NaBH4, MeOH, RT; (g) N-(cis-3-hydroxy-3-(2-oxoethyl)cyclobutyl)-2-naprittiamlde, NaBH(OAc)3, HOAc, DCM, RT, 4 h.