Abstract
A fatal case of meningitis due to Rhodotorula mucilaginosa in a 28 year-old HIV-negative male with a history of Hodgkin lymphoma who underwent salvage chemotherapy is presented. Reviewing the literature we identified 13 cases with central nervous system infection due Rhodotorula spp. The disease usually occurs in HIV negative immunosupressed middle-aged males. It takes the form of subacute or chronic meningitis accompanied by fever with an overall mortality of 46.2% despite antifungal therapy.
Keywords: Meningitis, Rhodotorula mucilaginosa, Lymphoma
1. Introduction
Over the last decade several emerging fungal pathogens were implicated in invasive diseases in immunocompromised patients [1]. Besides Candida spp. several other more rare yeasts have emerged to infect such individuals [2]. Rhodotorula is a ubiquitous saprobiotic yeast that can colonize and infect susceptible patients especially those with malignancy or other immunosuppression. Rhodotorula belongs to the family of Sporidiobolaceae of the phylum Basidiomycota. It forms spherical to ellipsoidal budding yeasts and may sometimes form rudimentary hyphae and small capsules [3]. Three species have been described as human pathogens; Rhodotorula glutinis, Rhodotorula minuta, and Rhodotorula mucilaginosa (formerly known as Rhodotorula rubra). In the largest review to date analyzing 128 cases of infection associated with this rare yeast, 87% of the cases had underlying immunosuppression, whereas fungaemia in the presence of a central venous catheter was the most commonly encountered clinical entity [5]. A more recent review reported similar findings with fungemia as the predominant clinical manifestation [6].
Herein we describe a rare case of fungal meningitis due to Rhodotorula spp. in an immunosuppressed individual with a hematologic malignancy and the challenges faced in the diagnosis and management of this pathogen.
2. Case
A 28 year old male individual presented to the Haematology Unit on day 0 with a 3-day history of headache and fever ≤38.5 °C along with dysphagia. He had a previous medical history of grade IIISB Hodgkin lymphoma diagnosed 20 months ago. The patient was in complete remission after 8 cycles of adriamycin, bleomycin, vinblastine and dacarbazine (ABVD) 17 months ago when investigation with a positron emission tomography (PET) scan was negative. Three months ago he relapsed and he re-presented with B symptoms, and disease foci in the lungs, liver, bones, and enlarged lymph nodes above and below the diaphragm (stage IVB). He then underwent salvage chemotherapy with 2 cycles of etoposide, methylprednisolone, cytarabine (Ara C) and cisplatin (ESHAP). Repeat PET scan on day -7 showed minimal residual involvement of the para-aortic lymph nodes and the patient was scheduled for autologous bone marrow transplantation.
The patient denied use of any medications including non steroidal anti-inflammatory agents, had no pets and no history of recent travel or any substance misuse. On examination he had photophobia and neurological symptoms including cranial nerve palsies of 6, 7 and 10, a pronator drift and Rombergs sign. He had difficulty swallowing and had a positive left Barre sign (pronator drift) as well as instability during the upright position. Fundoscopic evaluation was normal. Computed tomography of the brain was non diagnostic. Laboratory evaluation showed a WBC count of 10,800 c/mm3 (polymorphonuclear leukocytes: 75%, lymphocytes: 17%), a hemoglobin of 12 g/dl and a platelet count of 229×109/L. Urea and creatinine levels as well as liver function tests were within normal limits whereas C-reactive protein measurement was 0.7 mg/L (normal values<6 mg/L). A chest-X ray was normal.
Cerebrospinal fluid (CSF) examination on admission reviewed disclosed a yellow semi-turbid fluid with 175 cells/mm3 (lymphocytes: 93%, polymorphonuclear leukocytes: 3%), a glucose level of 35 mg/dl and a protein level of 128 mg/dl. Latex antigen testing for common bacterial meningitis pathogens on the CSF was negative. CSF Gram stain and Ziehl–Nielsen test was negative. CSF and blood cultures for common bacterial and acid fast bacilli (AFB) were performed and were negative. CSF PCR testing was negative for tuberculosis, Herpes simplex virus, Varicella Zoster virus, Epstein Barr virus, enterovirus, JC virus and Cytomegalovirus. Cryptococcal antigen testing both in serum and CSF was negative. Serological testing for HIV1/2, HTLV-I/II, toxoplasmosis, brucellosis and syphilis as well as Aspergillus galactomannan, Candida mannan and anti-mannan testing was negative. The patient had positive IgG but not IgM titers for HSV and cytomegalovirus. Cytological evaluation of the CSF found no evidence of malignant cells.
On admission, the patient was started on an empirical antimicrobial regimen of ampicillin, acyclovir, isoniazid, rifampin, ethambutol, pyrazinamide, moxifloxacin and dexamethasone. He continued to receive his trimethoprim-sulfamethoxazole (TMP-SMX) prophylaxis. He appeared to be responding over the next 2–3 days, remaining afebrile with improving focal neurology.
Repeat CSF testing on day 7 showed again a yellow semi-turbid fluid with 1798 cells/mm3 (lymphocytes: 95%), a glucose level of 36 mg/dl and a protein level of 176 mg/dl. The CRP was elevated to 120 mg/L. MRI imaging on the same day (Fig. 1) disclosed several foci of increased signal throughout the brain without hemorrhage (left and right frontal lobes, parietal and temporal lobes bilaterally and left occipital lobe, thalamus and right midbrain, pons, cerebellum, brainstem and cervical region of the spinal column) and leptomeningeal enhancement. Repeat CSF testing for bacterial pathogens and AFB were negative; however, the cytological examination found a few fungal forms (Fig. 2A). The patient was started on day 8 on a combination regimen of liposomal amphotericin B (450 mg/d) and flucytosine (2 g qid), and antituberculous regimens were discontinued. He continued to receive dexamethasone, acyclovir and prophylactic TMP/SMX.
Fig. 1.
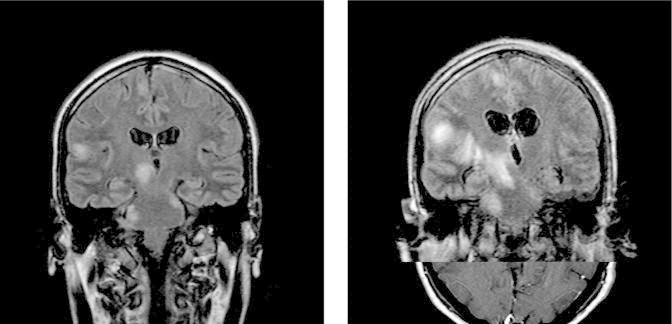
Several foci of increased signal throughout the brain on the first MRI on day 7 (left), and the repeat MRI on day 17 (right).
Fig. 2.
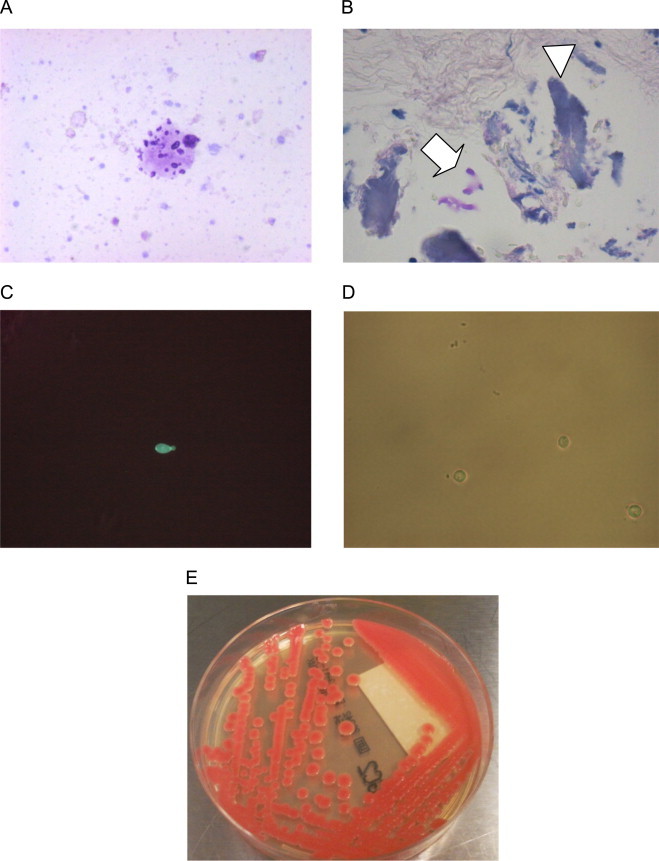
Cytology, histology and microbiology. (A) Fungal forms within proteinaceous material seen the cytological evaluation of the second CSF specimen obtained 7 days after the admission (Giemsa stain, 40×). (B) Focus of meningeal calcification (arrowhead) containing a few PAS (+) fungi (arrow), (PAS stain, 10×). (C) Fluorescence microscopy of biopsy specimen. (D) Optical microscopy of the yeast isolated after 4 weeks of incubation in liquid SAB (a capsule may be seen between the mother and the faint daughter cell). (E) The isolate of SAB agar.
A brain biopsy was performed on day 8. The CRP was 35 mg/L. In histology, few calcified foci were found within the dura mater, one of them containing a few PAS-positive fungi (Fig. 2B). Direct fluorescence microscopy of tissue specimen using 20% KOH with 0.25 mg/ml Blankophor P revealed few budding ellipsoidal blastoconidia of 4–6 μm in diameter (Fig. 2C). Tissue specimens were then inoculated in 15 ml liquid Sabouraud dextrose (SAB) medium and SAB agar and incubated for 4 weeks at both 30 °C and 37 °C.
The fever and neurological signs persisted despite therapy and a repeat MRI brain on day 17 depicted worsening and enlargement of the aforementioned brain lesions as well as enlargement of the lateral ventricles of the brain (Fig. 1). The patient was intubated for airway protection and was transferred to the medical intensive care unit (MICU). He developed nosocomial pneumonia secondary to aspiration and was treated with meropenem, colomycin (colistimethate sodium) and high-dose fluconazole (800 mg b.i.d.) on day 18 in addition to combination therapy of liposomal amphotericin B with flucytosine. His steroids were gradually reduced to 4 mg of dexamethasone per day on day 22. His CRP declined from 140 mg/L on day 17 to 20 mg/L on day 22 and procalcitonin levels were normal.
On day 32, when efforts to awaken failed, and further worsening of his neurological status with worsening anisocoria was noted. Diffuse brain edema and further enlargement of the ventricular system was noted on repeat brain imaging on the same day. A neurosurgical review concluded that the patient should be managed conservatively with mannitol and dexamethasone 32 mg per day. Electroencephalography showed a suppression of brain activity on day 34 with mydriasis and no eye reflex; repeat brain imaging showed diffuse decrease in cerebral flow, extensive brain edema and central tentorial herniation. The CRP further declined to reach normal levels on day 37 when the patient died. Autopsy permission was not granted.
On day 40 moist, glistening, smooth to mucoid, salmon pink yeast colony grew from liquid SAB of the biopsy specimen after 4 weeks of incubation (Fig. 2E). Direct microscopy showed round to ovoid budding yeasts with a small capsule (Fig. 2D). The Auxacolor identification system (profile number 75,471+14) confirmed the Vitek2 (Biomerieux, France) system identification of R. mucilaginosa/glutinis with good identification (92%) and negative nitrate and positive raffinose assimilation indicating R. mucilaginosa [3]. Identification was confirmed by sequencing the internal transcriber region with the universal primers ITS1 and ITS4 as previously described [4]. The closest hit in the genbank was R. mucilaginosa with 100% identity (Genbank accession number KM401434). Sensititre YeastOne antifungal susceptibility testing after 48 h incubation showed the following minimal inhibitory concentrations: amphotericin B 0.5 mg/l, flucytosine 0.06 mg/l, voriconazole 0.06 mg/l, itraconazole 0.12 mg/l and posaconazole 0.25 mg/l, fluconazole 32 mg/l and echinocandins anidulafungin, caspofungin and micafungin>8 mg/l.
3. Discussion
We present a rare case of Rhodotorula mucilaginosa associated central nervous system infection in a patient with a hematological malignancy. Rhodotorula spp. are very rarely implicated in human infections in susceptible hosts such as patients with solid or hematological malignancy or patients with HIV [1]. Six cases of Rhodotorula spp. associated central nervous system (CNS) infection (5 meningitis cases and 1 ventriculitis case in the presence of an intraventricular catheter) were presented in the largest review paper that evaluated published data from 1976 to 2001 [7–12] (Table 1). Two had HIV infection and 1 had an underlying acute leukemia. Five of these originally reported cases (4 meningitis and one ventriculitis) were considered to be healthcare-associated [5]. Together with the cases described in the original report by Tuon et al., we have identified in the literature (NCBI, PUBMED Database, accessed May 1st 2014) an additional 6 cases with central nervous system involvement (in total 13 cases with the one reported here) associated with Rhodotorula spp. (Table 1).
Table 1.
Cases of Rhodotorula CNS infections reported in the literature.
| N | Year | Author [Ref] | Yeast | Age | Gender | Clinical diagnosis | Underlying disease | Treatment | Outcome |
|---|---|---|---|---|---|---|---|---|---|
| 1 | 1976 | Pore RS [12] | R. mucilaginosa | 14 | Male | Meningitis | Acute leukemia | Amphotericin B (AMB) | Death |
| 2 | 1987 | Donald FE [8] | R. mucilaginosa | 32 | Female | Ventriculitis | Meningioma | 5-FC | Cure |
| 3 | 1996 | Gyaurgieva [9] | R. mucilaginosa | 39 | Male | Meningitis | HIV/AIDS | 5-FC, relapse, long term therapy with itraconazole | Cure |
| 4 | 1998 | Huttova M [10] | R. mucilaginosa | 13 | Male | Meningitis | Neuroblastoma | Miconazole | Cure |
| 5 | 1998 | Ahmed A [7] | R. mucilaginosa | 65 | Female | Meningitis | HIV/AIDS | Miconazole | Death |
| 6 | 2001 | Lanzafame M [11] | R. glutinis | 69 | Male | Meningitis | Immunocompetent, elderly | AMB | Cure |
| 7 | 2007 | Pamidimukkala U [16] | R. glutinis | 20 | Female | Meningoencephalitis | Systemic Lupus erythematosus | None | Death |
| 8 | 2007 | Thakur K [17] | R. mucilaginosa | 30 | Male | Meningitis | HIV/AIDS | AMB+5FC | Death |
| 9 | 2008 | Baradkar VP [22] | R. mucilaginosa | 36 | Male | Meningitis | HIV/AIDS | AMB followed by itraconazole | Cure |
| 10 | 2008 | Shinde RS [14] | R. glutinis | 35 | Male | Meningitis | HIV/AIDS | AMB | Cure |
| 11 | 2009 | Elias ML [13] | Rhodotorula spp | 53 | Male | Meningitis | Unknown | Not reported | Death |
| 12 | 2011 | Loss SH [15] | R. mucilaginosa | 58 | Male | Meningoencephalitis/endocarditis | Immunocompetent | Liposomal AMB | Cure |
| 13 | 2014 | Present study | R. mucilaginosa | 28 | Male | Meningitis | Hodgkin lymphoma | Liposomal AMB+5FC | Death |
The disease usually takes the form of subacute or chronic meningitis accompanied by fever, with a variable outcome [7,13,14]. The identification of microcalcifications in the brain biopsy of the case presented here is indicative of an underlying indolent course. One of the patients had both meningitis and infective endocarditis [15]. In a few instances, the disease was only discovered post-mortem [16]. In 8/11 cases (73%) with complete microbiological identification Rhodotorula mucilaginosa was the implicated yeast while in the rest Rhodotorula glutinis was isolated (Table 1). The median age of the affected individuals in the current analysis was 35 years old (IQR: 24–55.5 years) while 10/13 (76.9%) were male. Eight of the 13 patients (61%) were HIV negative and 5 (39%) were HIV positive (Table 1); in some instances the fungal meningitis due to Rhodotorula spp. was the opportunistic infection leading to the diagnosis of HIV/AIDS [17]. Six of the 13 patients died for an overall mortality of 46.2%.
Recently published guidelines recommended amphotericin B+flucytosine as the first line treatment for Rhodotorula infections with an associated overall mortality of 13.8% [2]. Seven patients in the present review of published cases with CNS Rhodotorula infection received amphotericin B-based regimens and 3 died (43%); two patients were treated with amphotericin B+flucytosine (Table 1). Previous studies have showed in vitro susceptibility of this yeast to amphotericin B preparations as well as flucytosine and their combination [18]. In vitro data suggest that fluconazole and echinocandins should not be routinely used against this pathogen. More data are needed about the potential therapeutic role of extended-spectrum azoles such as voriconazole, posaconazole and ravuconazole, as in vitro data shows some activity against Rhodotorula species [18].
Although Rhodotorula is a rapid growing yeast, the long incubation period required in our case before biopsy cultures became positive implying low fungal burden or dormancy; a state of the fungus that persists for long periods of time before reactivation and disease production during an immunosuppressant event. In our case, histology showed microcalcifications in the brain biopsy suggesting an indolent course of infection. Thus, the long incubation period required for Rhodotorula to grow may be explained by the presence of dormant cells. Although dormancy has been described for Cryptococcus spp. [19], it has not been previously reported for Rhodotorula spp.
The extensive brain edema in our patient could have been related to reactivation of the disease or the immune reconstitution inflammatory syndrome (IRIS) after the gradual discontinuation of dexamethasone therapy. IRIS has been recognized in non-HIV-infected patients recovering from immunosupression (e.g. withdrawal of immunosuppressants) and is associated with exposure to foreign antigens of an immune system with improved ability to respond and cause inflammation [20]. As in our case, patients with IRIS and cryptococcosis were more likely to have received prednisone [21]. Although a wide range of CNS-IRIS etiologies have been described including fungi like Cryptococcus, Coccidioides, Candida, and Sporothrix spp., Rhodotorula spp. has not been previously reported.
In conclusion, we described a rare case of fatal CNS infection associated with Rhodotorula mucilaginosa, a rare fungal pathogen. According to our review this yeast in most instances of CNS infection was associated with an underlying immunocompromised status and increased mortality. Further work is necessary to elucidate the pathogenesis of this rare fungus and better characterize the appropriate therapeutic options.
Conflict of interest
There are none.
Acknowledgments
None
References
- 1.Meletiadis J., Roilides E. Rare invasive fungal infections: epidemiology, diagnosis and management. Curr Fungal Infect Rep. 2013;4:351. [Google Scholar]
- 2.Arendrup M.C., Boekhout T., Akova M., Meis J.F., Cornely O.A., ESCMID Lortholary O. and ECMM joint clinical guidelines for the diagnosis and management of rare invasive yeast infections. Clin Microbiol Infect. 2014;20(Suppl. 3):S76–S98. doi: 10.1111/1469-0691.12360. [DOI] [PubMed] [Google Scholar]
- 3.De Hoog G.S., Guarro J., Gené J., Figueras M.J. 2nd ed. Centraalbureau voor Schimmelcultures; Utrecht, The Netherlands: 2000. Atlas of Clinical Fungi. [Google Scholar]
- 4.Nunes J.M., Bizerra F.C., Ferreira R.C., Colombo A.L. Molecular identification, antifungal susceptibility profile, and biofilm formation of clinical and environmental Rhodotorula species isolates. Antimicrob Agents Chemother. 2013;57(1):382–389. doi: 10.1128/AAC.01647-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Tuon F.F., Costa S.F. Rhodotorula infection. A systematic review of 128 cases from literature. Rev Iberoam Micol. 2008;25(3):135–140. doi: 10.1016/s1130-1406(08)70032-9. [DOI] [PubMed] [Google Scholar]
- 6.Garcia-Suarez J., Gomez-Herruz P., Cuadros J.A., Burgaleta C. Epidemiology and outcome of Rhodotorula infection in haematological patients. Mycoses. 2011;54(4):318–324. doi: 10.1111/j.1439-0507.2010.01868.x. [DOI] [PubMed] [Google Scholar]
- 7.Ahmed A., Aggarwal M., Chiu R., Ramratnam B., Rinaldi M., Flanigan T.P. A fatal case of Rhodotorula meningitis in AIDS. Med Health R Isl. 1998;81(1):22–23. [PubMed] [Google Scholar]
- 8.Donald F.E., Sharp J.F., Firth J.L., Crowley J.L., Ispahani P. Rhodotorula rubra ventriculitis. J Infect. 1988;16(2):187–191. doi: 10.1016/s0163-4453(88)94097-2. [DOI] [PubMed] [Google Scholar]
- 9.Gyaurgieva O.H., Bogomolova T.S., Gorshkova G.I. Meningitis caused by Rhodotorula rubra in an HIV-infected patient. J Med Vet Mycol. 1996;34(5):357–359. [PubMed] [Google Scholar]
- 10.Huttova M., Kralinsky K., Horn J., Marinova I., Iligova K., Fric J. Prospective study of nosocomial fungal meningitis in children--report of 10 cases. Scand J Infect Dis. 1998;30(5):485–487. doi: 10.1080/00365549850161494. [DOI] [PubMed] [Google Scholar]
- 11.Lanzafame M., De Checchi G., Parinello A., Trevenzoli M., Cattelan A.M. Rhodotorula glutinis-related meningitis. J Clin Microbiol. 2001;39(1):410. doi: 10.1128/JCM.39.1.410.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Pore R.S., Chen J. Meningitis caused by Rhodotorula. Sabouraudia. 1976;14(3):331–335. [PubMed] [Google Scholar]
- 13.Elias M.L., Soliman A.K., Mahoney F.J., Karam El-Din A.Z., El-Kebbi R.A., Ismail T.F. Isolation of cryptococcus, Candida, Aspergillus, Rhodotorula and nocardia from meningitis patients in Egypt. J Egypt Public Health Assoc. 2009;84(1–2):169–181. [PubMed] [Google Scholar]
- 14.Shinde R.S., Mantur B.G., Patil G., Parande M.V., Parande A.M. Meningitis due to Rhodotorula glutinis in an HIV infected patient. Indian J Med Microbiol. 2008;26(4):375–377. doi: 10.4103/0255-0857.43579. [DOI] [PubMed] [Google Scholar]
- 15.Loss S.H., Antonio A.C., Roehrig C., Castro P.S., Maccari JG. Meningitis and infective endocarditis caused by Rhodotorula mucilaginosa in an immunocompetent patient. Revista brasileira de terapia intensiva. 2011;23(4):507–509. [PubMed] [Google Scholar]
- 16.Pamidimukkala U., Challa S., Lakshmi V., Tandon A., Kulkarni S., Raju S.Y. Sepsis and meningoencephalitis due to Rhodotorula glutinis in a patient with systemic lupus erythematosus, diagnosed at autopsy. Neurol India. 2007;55(3):304–307. doi: 10.4103/0028-3886.35695. [DOI] [PubMed] [Google Scholar]
- 17.Thakur K., Singh G., Agarwal S., Rani L. Meningitis caused by Rhodotorula rubra in an human immunodeficiency virus infected patient. Indian J Med Microbiol. 2007;25(2):166–168. doi: 10.4103/0255-0857.32730. [DOI] [PubMed] [Google Scholar]
- 18.Diekema D.J., Petroelje B., Messer S.A., Hollis R.J., Pfaller M.A. Activities of available and investigational antifungal agents against Rhodotorula species. J Clin Microbiol. 2005;43(1):476–478. doi: 10.1128/JCM.43.1.476-478.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.O’Meara T.R., Alspaugh J.A. The Cryptococcus neoformans capsule: a sword and a shield. Clin Microbiol Rev. 2012;25(3):387–408. doi: 10.1128/CMR.00001-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bahr N., Boulware D.R., Marais S., Scriven J., Wilkinson R.J., Meintjes G. Central nervous system immune reconstitution inflammatory syndrome. Curr Infect Dis Rep. 2013;15(6):583–593. doi: 10.1007/s11908-013-0378-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Singh N., Lortholary O., Alexander B.D., Gupta K.L., John G.T., Pursell K. An immune reconstitution syndrome-like illness associated with Cryptococcus neoformans infection in organ transplant recipients. Clin Infect Dis. 2005;40(12):1756–1761. doi: 10.1086/430606. [DOI] [PubMed] [Google Scholar]
- 22.Baradkar V.P., Kumar S. Meningitis caused by Rhodotorula mucilaginosa in human immunodeficiency virus seropositive patient. Ann Indian Acad Neurol. 2008;11(4):245–247. doi: 10.4103/0972-2327.44561. [DOI] [PMC free article] [PubMed] [Google Scholar]


