Figure 4.
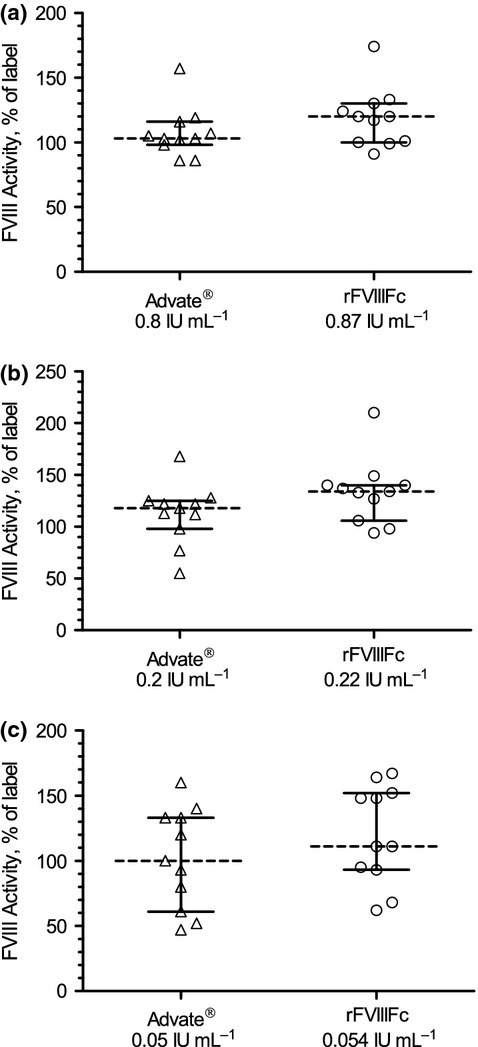
FVIII activity by chromogenic substrate assay showing a comparable distribution at all dose levels for rFVIIIFc and Advate®. (a) Advate® 0.8 IU mL−1, rFVIIIFc 0.87 IU mL−1; (b) Advate® 0.2 IU mL−1, rFVIIIFc 0.22 IU mL−1; (c) Advate® 0.05 IU mL−1, rFVIIIFc 0.054 IU mL−1. Box graphs represent median result, 25/75 percentile and minimum/maximum chromogenic substrate assay result as percentage of nominal (label) activity. Individual test results (n = 33) for each of the three FVIII levels were plotted. rFVIIIFc, recombinant factor VIII Fc fusion protein.
