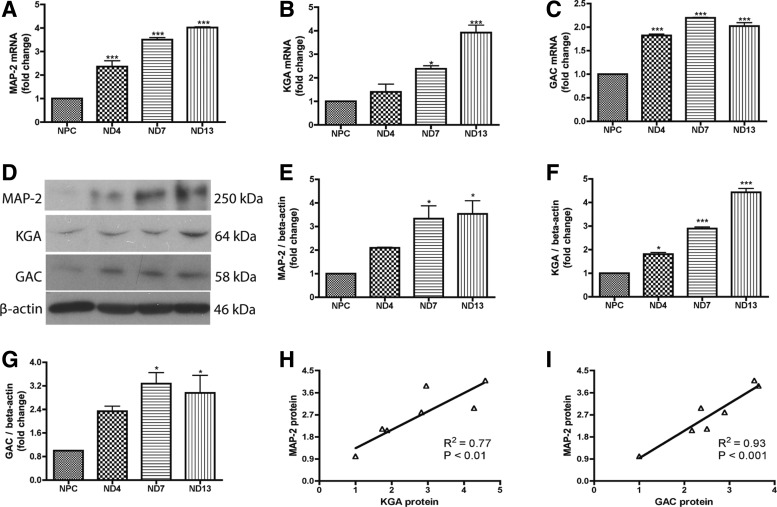
FIG. 1.
Glutaminase 1 (GLS1) isotypes, kidney-type glutaminase (KGA), and glutaminase C (GAC) were upregulated during neural progenitor cell (NPC) differentiation to neurons. (A–G) Human NPCs were exposed to the neuron differentiation medium for differentiation. (A–C) At 0, 4, 7, and 13 days after differentiation, mRNA was collected and expressions of microtubule-associated protein 2 (MAP-2) (A), KGA (B), and GAC (C) were analyzed using real-time reverse transcription–polymerase chain reaction (RT-PCR). Data were normalized to GAPDH and presented as fold change compared to NPCs. (D–G) In parallel, protein lysates were collected and analyzed by western blot for the expressions of MAP-2 (D, E), KGA (D, F), and GAC (D, G). β-Actin was used as loading control. Protein levels were normalized as a ratio to beta-actin after densitometric quantification and presented as fold change relative to NPCs. Data are shown as the mean±SEM of three independent experiments with three different donors. (H, I) Correlation of the gene expression levels of KGA (H) and GAC (I) with MAP-2 was determined by Spearman correlation. *P<0.05, ***P<0.001 compared with NPCs, n=3.