Figure 1.
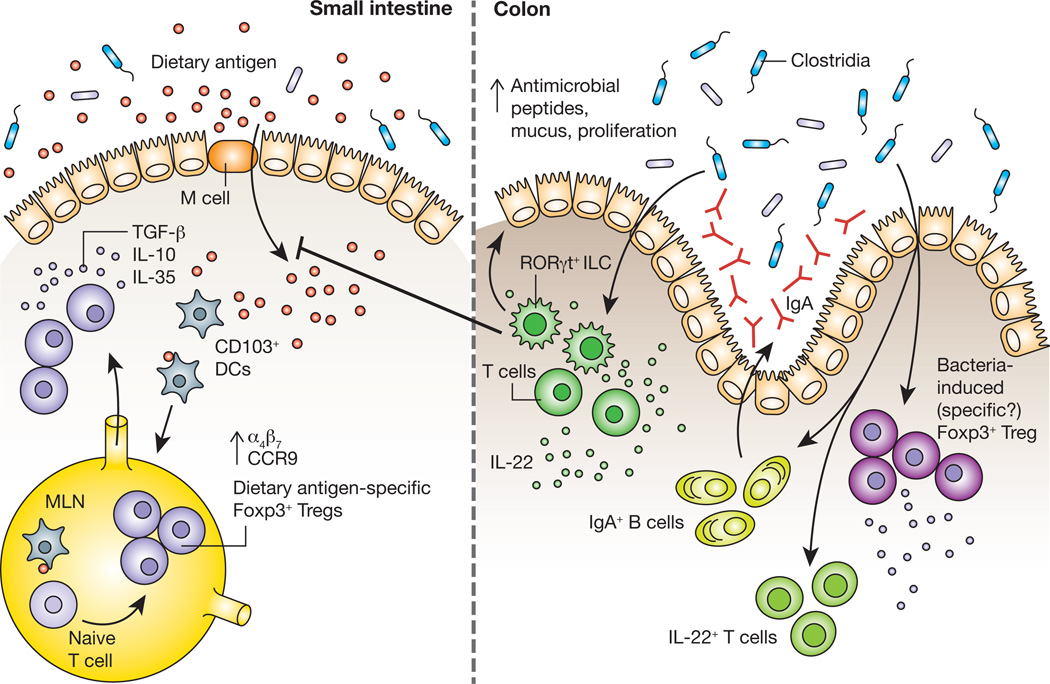
The process of maintaining non-responsiveness to food begins when dietary antigen crosses the intestinal epithelium. CD103+ DCs that have acquired antigen subsequently migrate to the mesenteric lymph node (MLN), where they encounter naïve T cells. Within the tolerogenic environment of the MLN, T cells differentiate into dietary antigen-specific Foxp3+ Tregs and upregulate expression of the gut-homing receptors α4β7 and CCR9, which drive their migration back to the intestinal lamina propria. Dietary antigen-specific Foxp3+ Tregs can then suppress aberrant responses to dietary antigens through the production of the cytokines TGF-β, IL-10 and IL-35. Work from our laboratory demonstrates that signals derived from Clostridia, a class of anaerobic spore-forming Firmicutes that resides in close proximity to the colonic epithelium are also required to prevent sensitization to food allergens. Studies in gnotobiotic mice showed that Clostridia colonization induces an early barrier protective response mediated by a surge in IL-22 production from RORγt+ innate lymphoid cells (ILCs). IL-22 acts on intestinal epithelial cells to promote the production of antimicrobial peptides and mucus and reduce uptake of dietary antigen into the systemic circulation. This initial barrier protective response is then reinforced by a bacteria-induced adaptive immune response that includes IL-22+ CD4+ T cells, an expansion of Foxp3+ Tregs in the colonic lamina propria and increased secretion of IgA into the intestinal lumen.