FIG. 2.
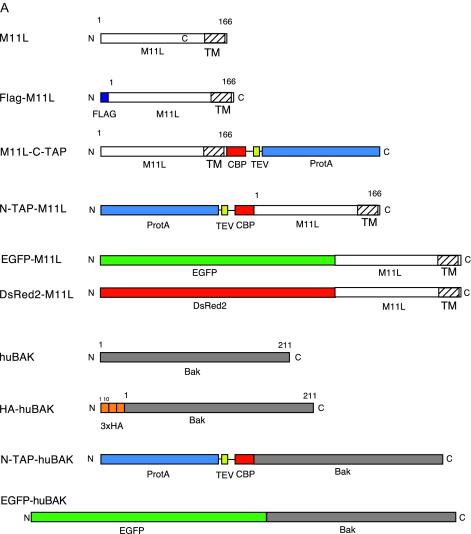

(A) M11L and huBak constructs utilized for this study. Constructs were either left untagged or fused to Flag, TAP, or HA epitope tags as indicated. The TAP cassette is composed of three components; the protein A (ProtA) IgG-binding sequence and the calmodulin binding protein (CBP) domain are separated by a TEV protease cleavage site. (B) Table listing the binding partners associated with M11L during Flag pull-down assays and identified by mass spectrometry. Interaction frequency, percentage of 20 independent transfections in which the protein was identified. (C) Immunoprecipitation of Flag-M11L and identification of associated proteins. Cell lysates were immunoprecipitated with anti-Flag agarose, and bound proteins were eluted with Flag peptide. Eluted proteins were resolved on a 4 to 15% Tris-HCl SDS-gel and stained with Coomassie blue. Lane 1, molecular weight markers; lane 2, immunoprecipitation from untransfected control HEK293T cells; lane 3, immunoprecipitation from cells transfected with Flag-M11L. Each of the bands in the second and third lanes was excised, digested with trypsin, and analyzed by mass spectrometry. (D) Typical output from mass spectrometry of peptide sequence run. A short, 10-amino-acid residue peptide within the complete Bak sequence was identified as a Bak peptide (lowercased letters).
