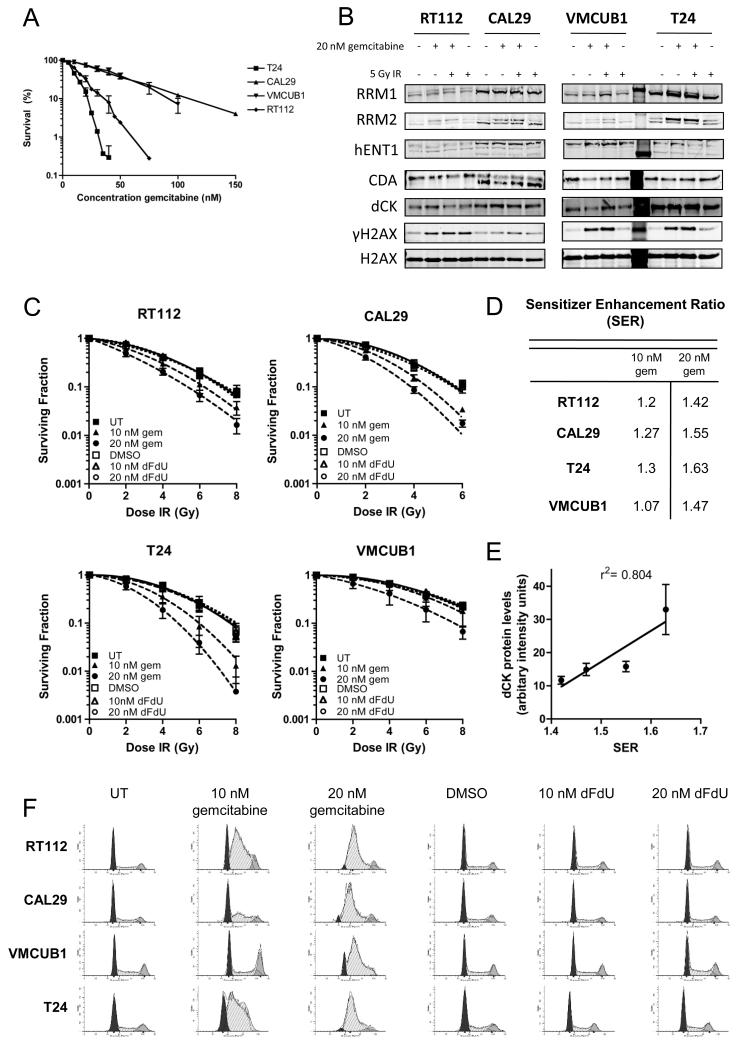
Figure 1. Low dose gemcitabine is a radiosensitiser in bladder cancer cells.
A) Clonogenic assay to determine gemcitabine cytotoxicity in a panel of bladder cancer cell lines. Subconfluent cells were treated with 20 nM gemcitabine prior to plating 600-2000 cells per dish, depending on cell line, and colony formation measured 2 weeks later.
B) Western blots for proteins involved in the uptake and metabolism of gemcitabine (n=3). RT112, CAL29, T24 and VMCUB1 bladder cancer cell lines were treated with 20 nM gemcitabine for 24 h and with 5 Gy IR where appropriate, collected 2 h post treatment and immunoblotted for RRM1 and RRM2, hENT1, CDA, and dCK. Equal loading was confirmed by blotting for total H2AX and DNA damage was confirmed by blotting for γH2AX.
C) Cells were treated as in A) and subsequently treated with IR immediately after plating. Colonies were counted 2 weeks after plating.
D) Sensitiser enhancement ratios at 10% survival. P values for comparisons were calculated using two-way ANOVA and were as follows: RT112: UT vs 10 nM gem P= 0.008, UT vs 20 nM gem P <0.0001; CAL29: UT vs 10 nM gem P= 0.004, UT vs 20 nM P <0.0001; T24: UT vs 10 nM gem P= 0.002, UT vs 20 nM gem P< 0.0001; VMCUB1: UT vs 10 nM gem P= 0.315, UT vs 20 nM gem P= 0.0013.
E) Correlation between dCK protein levels and SER. dCK protein levels were determined by densitometry of western blots from four independent experiments. These values were plotted against SERs obtained from clonogenic assays performed in triplicate.
F) Cell cycle analysis of gemcitabine and dFdU treated bladder cancer cell lines. Cells in exponential growth phase were incubated with gemcitabine or dFdU for 24 h prior to fixing and staining with propidium iodide.