FIG. 2.
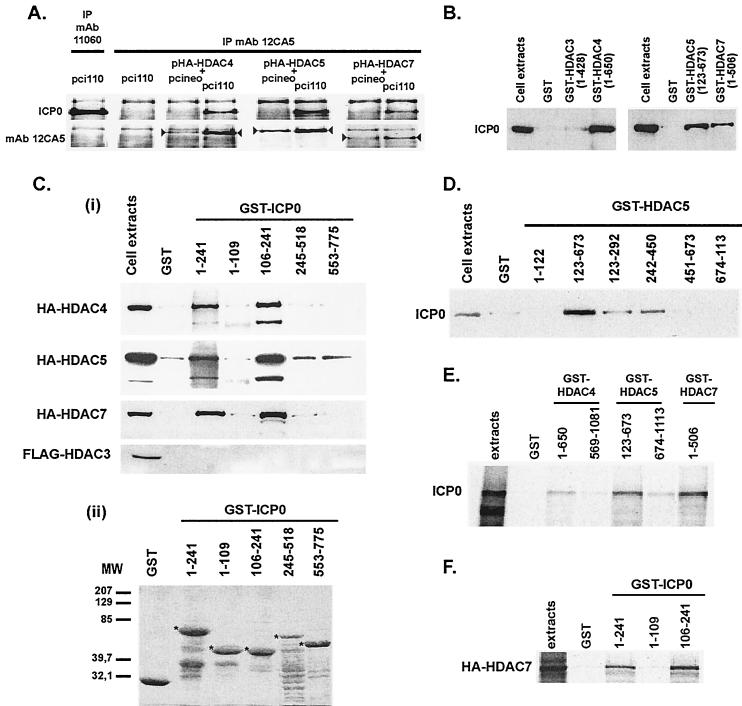
Physical interaction between ICP0 and class II HDACs and mapping of the interaction sites. (A) Coimmunoprecipitation of ICP0 with HA epitope-tagged class II HDAC4, -5, or -7. MAb 12CA5 was used for the immunoprecipitation (IP), and ICP0 was detected by Western blotting. Detection of the immunoprecipitated tagged-HDACs (arrowheads) was done as a control of HDAC expression. Immunoprecipitation of ICP0 with MAb 12CA5 (second upper left image) and detection of MAb 11060-immunoprecipitated ICP0 by MAb 12CA5 (first bottom left image) showed no cross-reactivity of ICP0 with this antibody. (B and Ci) GST pull-down experiments showing the capture of ICP0 (from ICP0-expressing cell extracts) (B) or tagged HDACs (from HDACs overexpressing cell extracts) (Ci) by GST-HDACs or GST-ICP0 fusion proteins, respectively. (Cii) Coomassie gel showing the expression of the GST-ICP0 fusion proteins (5 μg of proteins [✽]) used in panel Ci. (D) Mapping of the ICP0-interacting regions in HDAC5 protein by GST pull-down with GST-HDAC5 fusion proteins. (E and F) GST pull-down experiments with in vitro-synthesized ICP0 or HA-HDAC7, respectively. ICP0 and HA- or FLAG-tagged HDACs were detected in Western blots with MAbs 11060, 12CA5, or M5, respectively.
