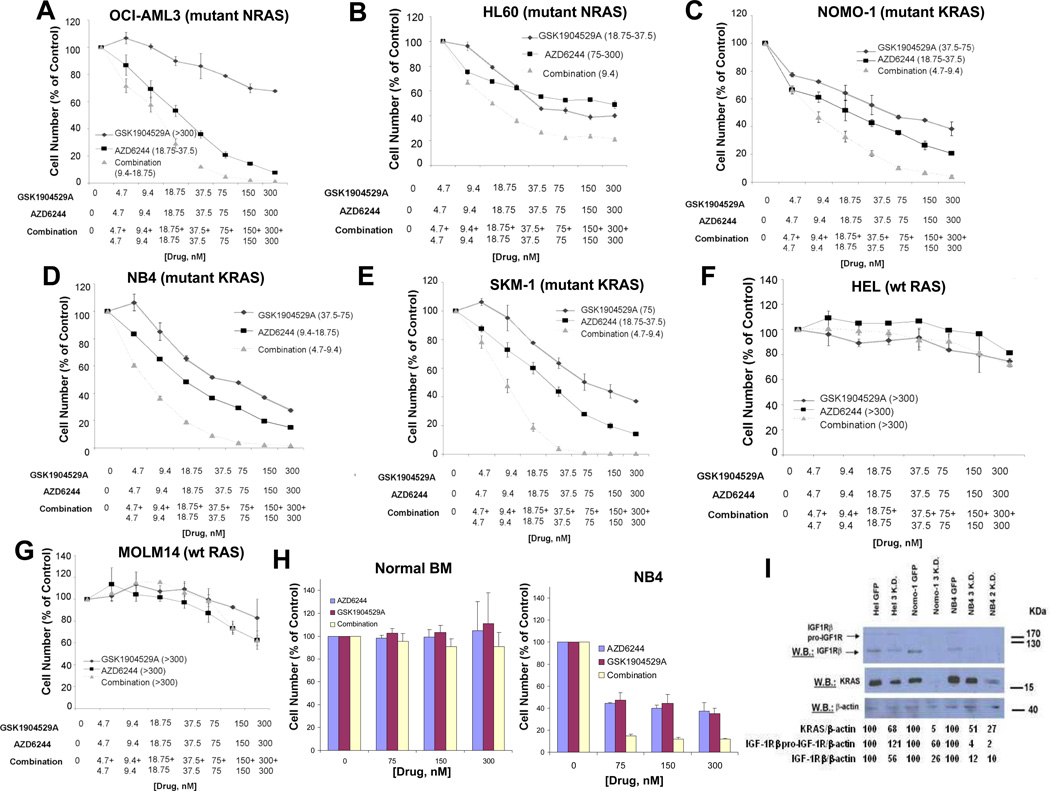
Figure 3. Combined effects of GSK1904529A and AZD6244 against human AML cells harboring wt or mutant RAS.
(A) Approximately 72-hr representative proliferation study performed with GSK1904529A and AZD6244, against mutant NRAS-expressing OCI-AML3 (n=3). (B) Approximately 48-hr proliferation study performed with GSK1904529A and AZD6244 against mutant NRAS-expressing HL60. (C–E) Approximately 72-hr representative proliferation studies performed with GSK1904529A and AZD6244 against NOMO-1 (KRAS G13D) (n=4), NB4 (KRAS A18D) (n=2), or SKM-1 (KRAS K117N) (n=2) cells. (F–G) Approximately 72-hr representative proliferation studies performed with GSK1904529A and AZD6244, against wt Ras-expressing Hel (n=2) and Molm14 cells. Shown in parentheses adjacent to figure legends for all graphs (A–G) are estimated IC50 values (nM) corresponding to individual drugs or drug combinations. (H) Shown as a normal control are the effects of two days of treatment using GSK1904529A and AZD6244, alone and combined, against mononuclear cells derived from normal donors cultured in DMEM+ 10% FBS (n=3). NB4 cells were tested in parallel as a positive control for drug stock integrity. (I) Effect of knockdown of KRAS on IGF1R expression in wt and mutant KRAS-expressing AML cells. Hairpins “2” and “3” were used for knockdown experiments.