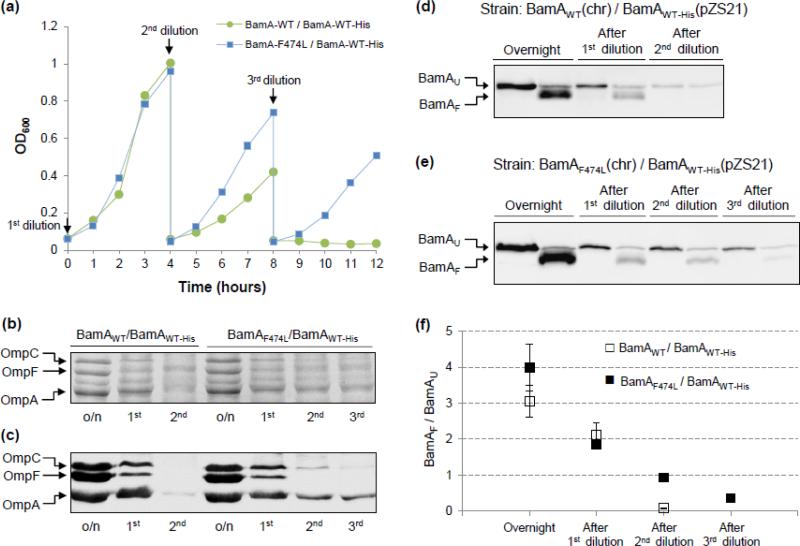
Fig. 4.
Effects of BamD depletion on bacterial growth, OMP levels, and BamA folding in strains partially diploid for bamA. (a) Overnight grown cultures in glycerol minimal medium were diluted three times and grown in a 37°C water bath as described in Fig. 1. BamD expression is repressed in glucose minimal medium. Cell densities were measured in a spectrophotometer at 600 nm. The untagged BamA proteins were expressed from the chromosome, while BamAWT-His was expressed from pZS21 (Kmr). Medium was supplemented with kanamycin to retain the pZS21 plasmid and with tetracycline to induce the expression of bamA from the plasmid replicon. Without tetracycline, the TetR repressor protein from a chromosomally localized zae::Tn10 inhibits the expression of bamA from the pZS21 plasmid. (b) and (c) SDS(urea)-PAGE and Western blot analyses of envelopes purified from glycerol-grown overnight (o/n) cultures and after three dilutions (1st, 2nd, and 3rd) in glucose minimal medium (Fig. 4a). Protein bands were visualized either after Coomassie blue staining (b) or Western blot analysis using OmpF/C/A antibodies (c). BamA compositions of the partially diploid strains are labeled on top. (d) and (e) BamA folding status was determined from envelopes obtained from cultures grown overnight in glycerol minimal medium and after dilutions in glucose minimal medium (Fig. 4a). Prior to SDS-PAGE analysis, SDS-solubilized samples were either left at room temperature (25°C) or heated (100°C). Detection of BamAWT-His was carried out by Western blot analysis using His-Probe. BamAU and BamAF refer to unfolded and folded forms of BamA, respectively. (f) Quantification of BamA folding. The graph shows the ratios of folded (BamAF) to unfolded (BamAU) species. Data were obtained from two independent experiments.