FIG. 3.
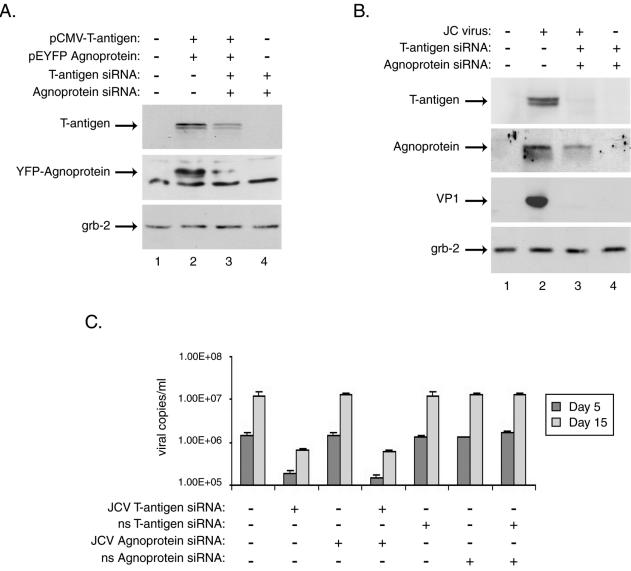
Treatment with siRNAs targeting JCV T antigen and agnoprotein abolishes their expression, as well as that of the JCV late protein VP1, in primary human astrocytes and affects viral replication in infected cells. Primary human fetal astrocyte preparations, transient transfections, siRNA treatments, and Western blotting were performed as described in the text and the legend to Fig. 1. (A) Whole-cell extracts from astrocytes transfected with expression plasmids for JCV T antigen, YFP-agnoprotein, or both were prepared from cultures 24 h after siRNA treatment and were analyzed by Western blotting for the presence of T antigen and agnoprotein as well as for the unrelated protein Grb-2. (B) Astrocyte cultures were infected with the JCV Mad-4 strain and were then transfected with siRNAs targeting JCV T antigen, agnoprotein, or both at days 1, 5, and 10 postinfection. Western blotting was performed on whole-cell extracts harvested at day 15 postinfection for the presence of JCV T antigen and agnoprotein as well as the viral late protein, VP1, and the cellular protein Grb-2. Supernatants collected from infected and siRNA-treated cells at days 5 and 15 postinfection were analyzed by quantitative real-time PCR for the presence of replicated JCV DNA essentially as described previously (17). The PCRs included JCV-specific forward and reverse primers (200 and 400 nM) representing nt 2393 to 2412 and 2468 to 2486 of the Mad-1 strain of JCV plus 200 nM JCV-specific probe (nt 2428 to 2458) fluorescently labeled at the 5′ and 3′ ends with FAM and BHQ1, respectively. Five microliters of cell culture supernatant was directly analyzed in triplicate in 50-μl reaction mixtures containing the above primers and probe in 1× TaqMan Universal Master Mix (Perkin-Elmer). Plasmid DNA containing the JCV genome was used to generate a standard curve against which the samples were analyzed using iCycler software (Bio-Rad).
