Abstract
Increasing mouse litter size [crowded litter (CL)] presumably imposes a transient nutrient stress during suckling and extends lifespan through unknown mechanisms. Chronic calorically restricted and rapamycin-treated mice have decreased DNA synthesis and mTOR complex 1 (mTORC1) signaling but maintained protein synthesis, suggesting maintenance of existing cellular structures. We hypothesized that CL would exhibit similar synthetic and signaling responses to other long-lived models and, by comparing synthesis of new protein to new DNA, that insight may be gained into the potential preservation of existing cellular structures in the CL model. Protein and DNA synthesis was assessed in gastroc complex, heart, and liver of 4- and 7-mo CL mice. We also examined mTORC1 signaling in 3- and 7-mo aged animals. Compared with controls, 4-mo CL had greater DNA synthesis in gastroc complex with no differences in protein synthesis or mTORC1 substrate phosphorylation across tissues. Seven-month CL had less DNA synthesis than controls in heart and greater protein synthesis and mTORC1 substrate phosphorylation across tissues. The increased new protein-to-new DNA synthesis ratio suggests that new proteins are synthesized more so in existing cells at 7 mo, differing from 4 mo, in CL vs. controls. We propose that, in CL, protein synthesis shifts from being directed toward new cells (4 mo) to maintenance of existing cellular structures (7 mo), independently of decreased mTORC1.
Keywords: slowed aging, crowded litter, proteostasis, deuterium, mTORC1
a progressive age-dependent reduction in protein turnover results in an accumulation of damaged proteins and propagates the development of the aging phenotype (36, 37). In tissues that have a limited proliferative capacity, such as heart and skeletal muscle, the ability to maintain functional proteins (i.e., proteostasis) has become a key outcome in aging research as a means to maintain homeostasis with age (1, 2). Thus, interventions that maintain protein stability could lead to slowed aging.
In proliferative tissues, growth is accomplished by cell replication, which requires the duplication of DNA and of cellular machinery through increased protein synthesis (reviewed in Ref. 16). In tissues with limited ability to proliferate (e.g., skeletal muscle), growth is accomplished by increased protein synthesis, which may be followed by recruitment of new DNA from satellite cell division to maintain the myonuclear domain (44). Since enzymatic capacity for protein repair is low within cells, damaged proteins must be removed and subsequently replaced through the synthesis of new protein (20, 26), a process that does not necessarily involve cell division or DNA recruitment. Therefore, the simultaneous assessment of DNA synthesis and protein synthesis could provide context as to whether the synthesis of new proteins are directed toward new cells or to maintaining existing cellular structures, a phenomenon consistent with slowed aging (11, 22, 23). Our laboratory has shown that, in lifelong caloric restriction (CR) (22, 23) and chronically rapamycin-treated mice (11), DNA synthesis is decreased. However, in long-lived models compared with controls, there are variable responses in protein synthesis based on the protein fraction, tissue, model, and sex. To date, no study, in a model of slowed aging or other, has considered protein synthetic rates in the context of DNA synthesis.
The mechanistic (formerly mammalian) target of rapamycin (mTOR) signaling pathway promotes growth by increasing protein synthesis and cellular cycling via two multiprotein complexes, mTORC1 and mTORC2 (18). CR (17, 46) and chronic rapamycin administration (14, 30, 45) suppress mTORC1 activity and increase lifespan and multiple indexes of health in various species. Although it has been proposed that a decrease in mTOR signaling is a key mechanism of slowed aging (18), it is unclear whether decreased mTOR signaling is necessary for slowed aging.
The nutritional environment of the developing fetus can have a profound impact on gene expression throughout the lifespan of the offspring, affecting health and longevity (8, 13, 40). Furthermore, changing litter size or postnatal maternal protein intake can change offspring lifespan, suggesting that some degree of genomic plasticity also remains during the postnatal suckling period (8, 12, 19, 38, 39). For example, increasing litter size by 50% for the first 3 wk of life, a model termed crowded litter (CL), extends mean and maximal lifespan (39). The increase in litter size presumably imposes a CR period until the pups are weaned and given free access to food (33, 39). How a transient nutrient stress at such a young age extends lifespan is unknown. We hypothesized that, like other long-lived models previously investigated in our laboratory (11, 22, 23), CL mice would have decreased DNA synthesis and mTORC1 signaling but maintained protein synthesis. In addition, we hypothesized that if new protein synthesis was considered in relation to new DNA synthesis, there would be an increase in the ratio within the CL model, indicative of greater preservation of existing protein structures.
METHODS
Animals and Treatments
All procedures and conditions at the animal care facility meet or exceed the standards for animal housing as described in the Animal Welfare Act regulations and the Guide for the Care and Use of Laboratory Animals and were approved by the University Committee on Use and Care of Animals at the University of Michigan. We used the genetically heterogeneous offspring of CB6F1 female and C3D2F1 male (UM-HET3) mice, which have been well characterized for the development of the CL model (38, 39).
Routine veterinary care was provided by the Biomedical Science Research Building staff. UM-HET3 litters were culled to eight, and an additional four mice from separate litters were added, resulting in a 50% increase in litter size (39). Control (Con) UM-HET3 mice were maintained in a litter size of eight pups. After the 3-wk suckling period, female mice were weaned onto a chow diet, and CL and Con were placed in their own respective cages (4 mice per group per cage) with ad libitum access to food and water. At 4 mo (not weight stable, still growing) and 7 mo of age [weight stabilizing, and same age as our previous investigations in long-lived models (11, 22, 39)], CL (n = 4, per age group) and Con (n = 4, per age group) mice received deuterium-labeled water (2H2O) for 2 wk. Animals received an intraperitoneal injection of 99% enriched 2H2O to enrich the body water pool (assumed 60% of body wt) to 8% (22, 28). Animals then received 8% 2H2O in their drinking water with ad libitum access for the next 2 wk. After the labeling period and following an overnight fast (7 mo only), mice were euthanized using a CO2 overdose according to the AVMA Guidelines on Euthanasia. Complete loss of pedal reflexes was confirmed before tissues were collected. The posterior aspect of the distal hindlimbs [gastroc complex: gastrocnemius, soleus, and plantaris (i.e., mixed skeletal muscle)], heart, liver, bone marrow from the tibia, via flushing the cavity with PBS, and plasma, via blood from cardiac puncture, were taken and immediately frozen in liquid nitrogen for later analysis.
The 4-mo mice were not fasted the night before being euthanized. Therefore, to assess mTORC1 signaling at a younger age, an additional cohort of ∼3-mo CL and Con (n = 4, respectively) mice were euthanized following an overnight fast, and gastroc complex and liver were harvested as already reported.
Protein Isolation
We assessed protein synthesis in three subcellular fractions: mitochondrial enriched (Mito), cytosolic (Cyto), and mixed (Mix). Mix contains nuclei, plasma membranes, and, in the case of skeletal muscle and heart, contractile proteins. Cyto contains all other intracellular components with the exception of mitochondria, which is contained in the Mito fraction. Tissues were fractionated according to our previously published procedures (11, 22, 23, 31). Briefly, tissues (25–60 mg) were homogenized 1:10 in isolation buffer (100 mM KCl, 40 mM Tris·HCl, 10 mM Tris base, 5 mM MgCl2, 1 mM EDTA, 1 mM ATP, pH 7.5) with phosphatase and protease inhibitors (HALT, Thermo Scientific, Rockford, IL) using a bead homogenizer (Next Advance, Averill Park, NY). After homogenization, subcellular fractions were isolated via differential centrifugation as previously described (11, 22, 23). Once fraction pellets were isolated and purified, 250 μl 1 M NaOH was added, and pellets were incubated for 15 min at 50°C and 900 rpm.
DNA Isolation
Whole tissue DNA synthesis in skeletal muscle, heart, and liver was assessed according to procedures previously described (5, 11, 22, 23, 28). Approximately 75 ng/μl (heart and gastroc complex) and 400ng/μl (liver) of total DNA was extracted from ∼15 mg tissue (QiAamp DNA mini kit Qiagen, Valencia, CA, USA). DNA for the precursor pool was obtained from bone marrow. DNA from bone marrow was isolated by extracting ∼300 mg from the tibial bone marrow suspension which was centrifuged for 10 min at 2,000 g. DNA from the resulting bone marrow pellet was extracted the same as tissue samples already described.
Sample Preparation and Analysis of Analytes Via GC-MS
Protein.
Protein was hydrolyzed by incubation for 24 h at 120°C in 6 N HCl. The hydrolysates were ion exchanged, dried under vacuum, and resuspended in 1 ml of molecular biology grade H2O. Suspended samples (500 μl) were derivatized [500 μl acetonitrile, 50 μl 1 M K2HPO4 (pH 11), and 20 μl pentafluorobenzyl bromide (Pierce Scientific, Rockford, IL)], sealed, and incubated at 100°C for 1 h. Derivatives were extracted into ethyl acetate. The organic layer was removed and dried by N2 followed by vacuum centrifugation. Samples were reconstituted in 1 ml of ethyl acetate and then analyzed.
The pentafluorobenzyl-N,N-di(pentafluorobenzyl) derivative of alanine was analyzed on an Agilent 7890A GC coupled to an Agilent 5975C MS, as previously described (11, 22, 23, 32). The newly synthesized fraction (f) of proteins was calculated from the true precursor enrichment (p) using plasma analyzed for 2H2O enrichment and adjusted using mass isotopomer distribution analysis (MIDA) (5). Protein synthesis was calculated as the ratio of 2H2O-labeled to unlabeled alanine (5) bound in proteins over the entire labeling period and expressed as fraction new in 2 wk.
Body water.
To determine body water enrichment, 125 μl of plasma was placed into the inner well of an o-ring screw-on cap and placed inverted on a heating block overnight. Two microliters of 10 M NaOH and 20 μl of acetone was added to all samples and to 20 μl 0–20% 2H2O standards and capped immediately. Samples were vortexed at low speed and left at room temperature overnight. Extraction was performed by the addition of 200 μl of hexane. The organic layer was transferred through anhydrous Na2SO4 into GC vials and analyzed via EI mode using a DB-17MS column.
DNA.
Determination of 2H incorporation into purine deoxyribose (dR) of DNA from whole tissue and bone marrow was performed as described previously (5, 11, 22, 32). Briefly, isolated DNA was hydrolyzed overnight at 37°C with nuclease S1 and potato acid phosphatase. Hydrolysates were reacted with pentafluorobenzyl hydroxylamine and acetic acid and then acetylated with acetic anhydride and 1-methylimidazole. Dichloromethane extracts were dried, resuspended in ethyl acetate, and analyzed by GC-MS as previously described (11, 22, 32). The fraction new in 2 wk was calculated by comparison with bone marrow (representing an essentially fully turned over cell population and thus the precursor enrichment) in the same animal (11, 22, 23).
New Protein-to-New DNA Synthesis Ratio
From the synthesis rates of protein and DNA, we calculated the new protein-to-new DNA synthesis ratio. The rationale is that this ratio illustrates how much protein is made in relation to the rate of new DNA synthesis during the labeling period, giving insight into whether new proteins are made in either existing or new cells.
Western Blotting
A portion of the Cyto fraction from 7-mo and the additional 3-mo-fasted cohort was used for Western blot analysis. Protein concentration was determined using a bicinchoninic acid assay (Thermo Fisher, Rockford, IL). Samples were diluted to the same concentration, boiled with Laemmli buffer, and then 30–45 μg of protein was separated using 10% SDS-PAGE at 100 V. Proteins were transferred at 4°C (100 V for 75 min in 20% wt/vol methanol, 0.02% wt/vol SDS, 25 mM Tris base, 192 mM glycine, pH 8.3) to nitrocellulose paper and incubated in 5% nonfat dry milk in Tris-buffered saline with Tween 20 (TBST) for 1 h. Antibodies were purchased from Cell Signaling Technologies [Boston, MA; rpS6 phospho-Ser235/236 no. 4858, rpS6 total no. 2217, eukaryotic initiation factor 4E binding protein-1 (4E-BP1) phospho-Thr37/46 no. 9459, 4E-BP1 total no. 9452], or β-tubulin no. sc-5274 (Santa Cruz Biotechnology, Santa Cruz, CA). Blots were incubated overnight with primary antibodies diluted 1:500 (skeletal muscle rpS6), 1:1,000 (all others), 1:2,000 (liver rpS6), or 1:500 (β-tubulin). Blots were washed in TBST and incubated with anti-rabbit or anti-mouse (β-tubulin) HRP-conjugated secondary antibodies diluted 1:5,000 in 5% milk with subsequent chemiluminescence detection (West Dura; Pierce, Rockford, IL). Images were captured and densitometry was analyzed by UVP Bioimaging system (Upland, CA). Blots were probed for phosphorylated proteins first, placed in stripping buffer (GM Biosciences, Rockville, MD), and then reprobed for total proteins. Equal loading was verified using Ponceau S staining and β-tubulin. Due to undetectable rpS6 in skeletal muscle homogenate, a portion of the homogenate was acetone precipitated and then analyzed via Western blotting. Phosphorylated proteins were expressed as a ratio with total.
Statistics
Statistical analysis was performed using Prism v.4.0c (GraphPad Software, La Jolla, CA). Comparisons between treatment groups and protein synthesis and new protein/new DNA synthesis ratio data in the various subcellular fractions were assessed by two-way ANOVA. DNA synthesis and Western blot data were assessed via two-sided Student's t-test. Variances were normalized via log transformation where necessary. Significance was set at P < 0.05, and P values of ≤0.10 are noted. Data are presented as means ± SE.
RESULTS
Protein and DNA Synthesis
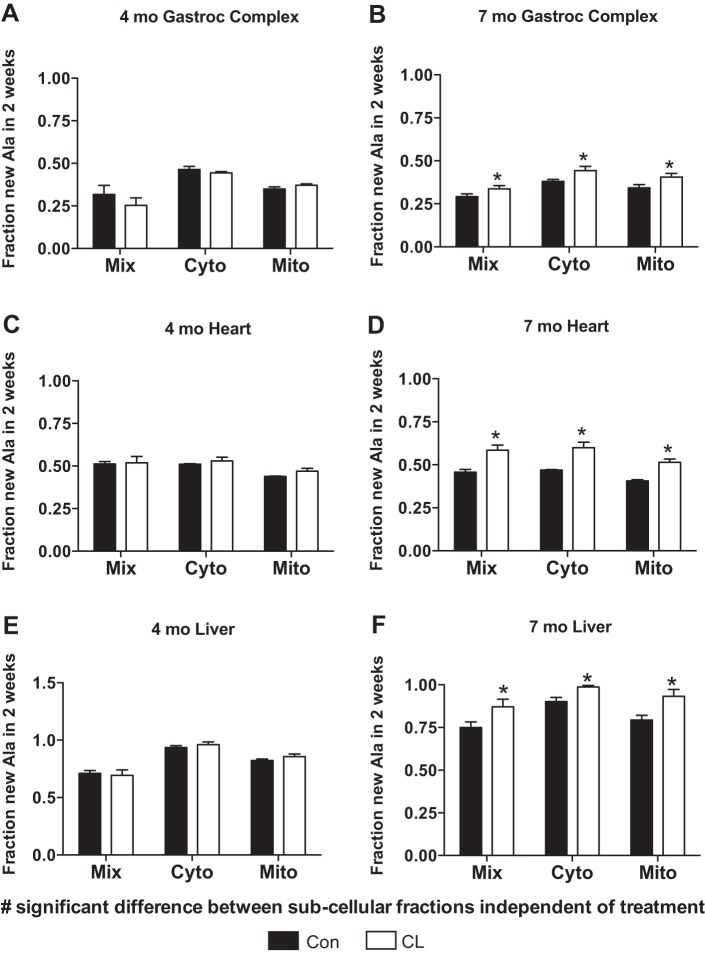
Body weight at weaning was significantly reduced in CL compared with Con mice (Fig. 1), suggesting that CL mice were calorically restricted during suckling (33, 39). However, there were no differences in body weight at either 4 or 7 mo of age (Fig. 1). In the gastroc complex, heart, and liver of 4-mo CL mice, there were no differences in protein synthesis within the Mix, Cyto, or Mito fractions compared with 4-mo Con (Fig. 2, A, C, and E). By 7 mo of age, there was a dramatic change, in the gastroc complex, heart, and liver (Fig. 2, B, D, and F) all having significantly greater rates of protein synthesis in Mix, Cyto, and Mito fractions compared with 7-mo Con (Fig. 2, B, D, and F). Independent of treatment, protein synthesis was significantly different between subcellular fractions in all tissues and ages (Fig. 2, A–F).
Fig. 1.
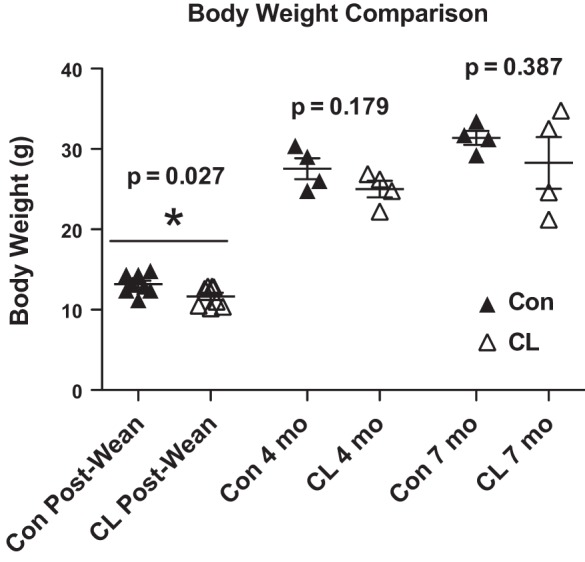
Body weight comparison of crowded-litter (CL) and control (Con) mice postweaning, at 4 mo and at 7 mo of age. Postweaning CL mice weighed significantly less than normal-litter-size Con mice. There was no difference in body weight between CL and Con mice at 4 or 7 mo of age; n = 8 per group for postweaning, n = 4 per group in 4-w and 7-mo-old cohorts. *P < 0.05 for CL vs. Con.
Fig. 2.
Protein synthesis in gastroc complex (gastrocnemius, soleus, and plantaris, i.e., mixed skeletal muscle), heart, and liver over 2 wk in 4-mo and 7-mo CL and Con. There were no differences in protein synthesis between groups in any tissue between 4-mo CL and 4-mo Con (A, C, and E). Protein synthesis was significantly higher in all tissues and fractions in 7-mo CL vs. 7-mo Con (B, D, and F); n = 4 per group and fraction except Con liver Mix n = 3 because of an unquantifiable peak on the GC. Two values in liver 7-mo CL Cyto and Mito were fully turned over (i.e., 100% new) after 2 wk, and so calculations were performed with a synthesis value of 1.00 for those animals. *P < 0.05 for CL vs. Con; no. P < 0.05 difference between pooled (Con and CL) fractions in all tissues at both 4 and 7 mo.
DNA synthesis was significantly greater in the gastroc complex of 4-mo CL mice compared with Con (Fig. 2A). DNA synthesis in heart trended greater in 4-mo CL compared with 4-mo Con but did not reach statistical significance (P = 0.070; Fig. 3A). Liver DNA synthesis in 4-mo CL was not different from that of 4-mo Con (Fig. 3A). At 7 mo, CL mice had significantly lower rates of DNA synthesis in the heart but not in gastroc complex and liver (Fig. 3).
Fig. 3.
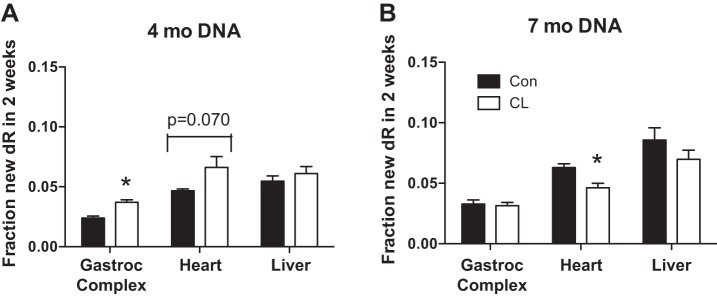
DNA synthesis in gastroc complex, heart, and liver over 2 wk in 4-mo and 7-mo CL and Con. A: gastroc complex DNA synthesis was significantly greater in 4-mo CL than in 4-mo Con, heart DNA synthesis in 4-mo CL tended to be greater (P = 0.070) than 4-mo Con, and there were no differences in liver DNA synthesis between 4-mo animals. B: in 7-mo animals, DNA synthesis was significantly less in CL heart than in Con, and no significant differences were observed between 7-mo CL and 7-mo Con in gastroc complex or liver. DNA synthesis rates in heart tissues from 4-mo mice were log transformed to normalize variance; n = 4 in both ages and groups except that in 7-mo Con gastroc complex and 7-mo CL gastroc complex and heart n = 3 due to one data point being excluded as an outlier. *P < 0.05 for CL vs. Con.
New Protein/New DNA Synthesis Ratio
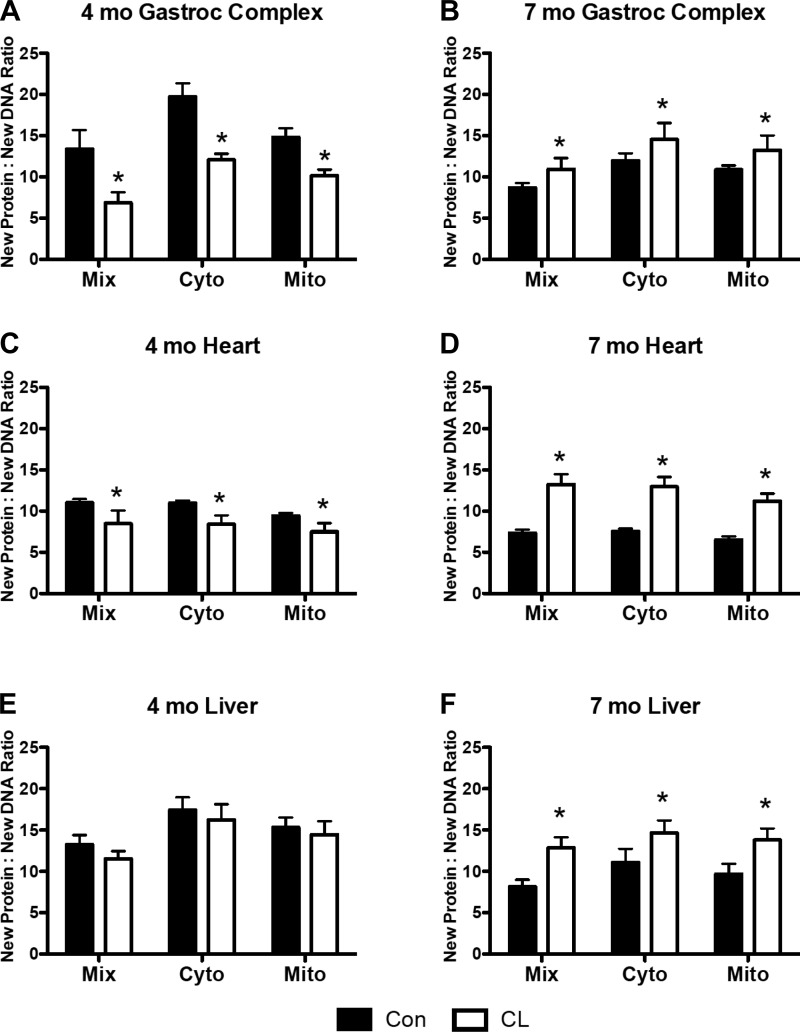
In the 4-mo CL, the new protein/new DNA synthesis ratio was significantly decreased compared with 4-mo Con across subcellular fractions in both the gastroc complex and heart (Fig. 4, A and C). At 4 mo, in the liver there was no difference between Con and CL in the new protein/new DNA synthesis ratio (Fig. 4E). However, at 7 mo, the new protein/new DNA synthesis ratio was significantly increased across subcellular fractions in gastroc complex, heart, and liver (Fig. 4, B, D, and F).
Fig. 4.
New protein-to-new DNA synthesis ratios in gastroc complex and heart of CL and Con. At 4 mo, there was a significant decrease in the new protein/new DNA ratio in CL compared with Con in Mix, Cyto, and Mito fractions in both gastroc complex and heart (A and C) but no change in any fraction between groups in liver (E). At 7 mo, there was a significant increase in the new protein/new DNA ratio in Mix, Cyto, and Mito fractions in gastroc complex, heart, and liver of CL compared with Con (B, D, and F); n = 4 per group, except 7-mo gastroc complex Con and CL and 7-mo heart CL n = 3. See legends for Figs. 1 and 2. *P < 0.05 for CL vs. Con.
mTORC1 Signaling
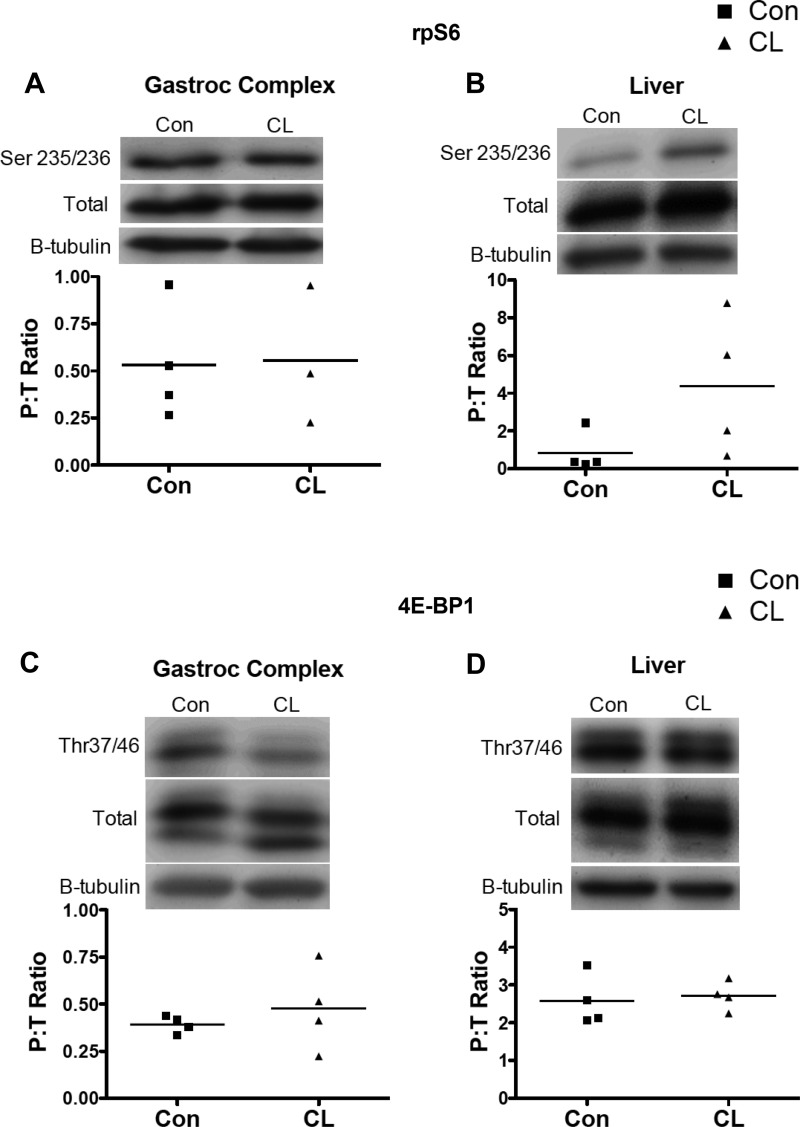
At 3 mo (additional fasted cohort), there were no differences between rpS6 and 4E-BP1 phosphorylation between groups in either gastroc complex or liver (Fig. 5). At 7 mo, phosphorylation of rpS6 was significantly increased in heart and liver (Fig. 6, B and C). Phosphorylation of 4E-BP1 was significantly increased in gastroc complex (Fig. 6D). Although it did not reach statistical significance, 4E-BP1 in heart (Fig. 4E) was P = 0.055.
Fig. 5.
Western blotting of mTOR complex 1 (mTORC1) substrates rpS6 and 4E-BP1 in fasted 3-mo CL mice. There were no differences between 3-mo CL and Con in rpS6 or 4E-BP1 phosphorylation in gastroc complex (A and C) or liver (B and D). Data are expressed as a ratio of phosphorylated to total protein. β-Tubulin is shown to verify equal loading of protein. In gastroc complex rpS6, CL n = 3 because one band was not visible to assess and thus was excluded to avoid misrepresenting the data; n = 4 in all other groups.
Fig. 6.
A–F: Western blotting of mTORC1 substrates rpS6 and 4E-BP1 in 7-mo CL mice. rpS6 phosphorylation was significantly greater in heart and liver in 7-mo CL than in Con (B and C). 4E-BP1 phosphorylation was significantly greater in gastroc complex and tended to be greater in heart (P = 0.055) in 7-mo CL vs. 7-mo Con (D and E). Data are expressed as ratio of phosphorylated to total protein. β-Tubulin is shown to verify equal loading of protein. Liver rpS6 was log transformed to normalize variance; n = 4 per group. *P < 0.05 for CL vs. Con.
DISCUSSION
Overview of Primary Findings
We tested the hypothesis that, compared with Con, CL mice would have decreased DNA synthesis and mTORC1 signaling, and maintained protein synthesis rates. Furthermore, we hypothesized that when the synthesis of new proteins was compared with the synthesis of new DNA there would be changes in the CL model that indicated a greater preservation of existing cellular structures. At 4 mo of age, protein synthesis was not different between CL and Con in any tissue but DNA synthesis was significantly increased in gastroc complex and trended in the heart of CL compared with Con. At 7 mo of age, however, protein synthesis rates were significantly greater in all tissues and fractions, whereas DNA synthesis was significantly less in the heart of CL than in Con. By comparing the synthesis of new protein to the synthesis of new DNA, we present novel insight into the potential destination of new proteins in long-lived CL mice. We demonstrate for the first time that, compared with normal litter sized controls, the transient nutrient stress of a 50% increase in litter size leads to alterations in the new protein/new DNA ratio later in life, suggesting a switch in which fewer of the new proteins synthesized are directed toward new cells and more are directed toward the maintenance of existing cellular structures. Finally, we report the novel finding of tissue-dependent increases in mTORC1 activity at 7 mo compared with no difference at 3 mo in the long-lived CL mice, suggesting that a decrease in mTORC1 may not be requisite for lifespan extension in CL.
Postnatal Feeding and Growth
Gene expression during in utero development can be profoundly affected by maternal nutrition, having lifelong effects on the healthspan and longevity of offspring (8, 13, 40). Changes in lifespan and healthspan through litter reduction in rats (3–4 pups/litter) (12) as well as litter enlargement (i.e., CL) experiments in mice (38, 39) suggest that nutritional status during early development can also have long-lasting effects. For example, reducing litter size increases pups' caloric intake, leading to a vast array of pathologies associated with poor health (12). Alternatively, CL extends mean and maximal lifespan (39) and upregulates the transcription of some phase I xenobiotic enzymes (38), which are protective against environmental toxins, and improves multiple markers of metabolic health (33). However, it is not known how an increase in litter size affects the synthetic processes that contribute to growth and increased lifespan and healthspan.
The use of 2H2O to measure DNA synthesis is reflective of S-phase synthesis, not DNA repair or random incorporation (6, 28). In our previous studies using models of slowed aging (11, 22, 23) and human exercise (32), we repeatedly demonstrated synthesis of new DNA in tissues comprised predominantly of postmitotic cells (i.e., heart and skeletal muscle). In the present study, we found changes in DNA synthesis within tissues comprised predominantly of postmitotic cells that is consistent with our previous investigations (11, 22, 23, 32). Therefore, we propose two possibilities for measurable changes in DNA synthesis in postmitotic tissues: 1) there is some mitotic ability in cardiac myocytes and skeletal muscle myofibers that are not fully appreciated; or 2) there are other cellular sources of new DNA in these tissues that change based on our interventions. For example, cardiomyocytes have some ability to replicate their own DNA (4, 35) or incorporate DNA from other neighboring cell types (3, 29). Furthermore, regulation of cardiac DNA synthesis may vary by stage of development (42), which is supportive of the varying age-dependent DNA synthesis rates in CL heart. Similarly, skeletal muscle is primarily made up of postmitotic, multinucleated myofibers but also contains resident proliferative satellite cells, pericytes, interstitial cells, and myoendothelial cells (10, 25, 47). When myofiber size increases, requiring an increase in protein synthesis, there is an increase in satellite cell recruitment (i.e., new DNA) to stabilize the myonuclear domain (32, 41), which isotopic labeling reflects (27). Previous work in exercising humans from our laboratory suggest that it is unlikely that mitochondrial DNA synthesis contributes significantly to our measurement of total DNA synthesis in skeletal muscle using 2H2O (32), although there could be a species difference in mice. Therefore, the increase in DNA synthesis in skeletal muscle of 4-mo CL may indicate a period of catch-up growth. Importantly, we assessed DNA synthesis by extracting DNA from whole tissue. Thus, the age- and tissue-dependent changes we present here are likely the summation of the various cell types within multiple, primarily postmitotic, tissues of the long-lived CL mouse model. Collectively, our current data in CL mice provide novel insights into growth regulation across whole tissue at different ages.
Insights from Simultaneous Assessment of Protein and DNA Synthesis
Low enzymatic capacity for protein repair within cells means that damaged proteins must be removed and replaced through synthesis of new proteins (20, 26). In light of our previous investigations in long-lived models, we posit that maintaining rates of protein synthesis while DNA synthesis is decreased suggests that newly synthesized proteins are made primarily in existing cells, which may promote the maintenance of existing cellular structures (i.e., proteostasis), contributing to increased lifespan and healthspan (2, 11, 22, 23). In contrast to our investigations into long-lived CR and chronic rapamycin-fed mouse models of the same age (11, 22), the 7-mo long-lived CL mice had increased protein synthesis in all tissues and subcellular fractions in gastroc complex, heart, and liver. It is important to note that the CL model is generated from mice of the same heterogeneous background (UM-HET3) as in our previous investigation of chronic rapamycin feeding (11); thus, strain differences cannot account for their differing response in protein synthesis. The elevated rates of mitochondrial protein synthesis in 7-mo CL, indicative of mitochondrial biogenesis (21), is consistent with our previous investigations and hypothesized to be key to slowed aging (11, 22, 24). Therefore, determining whether the increase in protein synthesis in 7-mo CL was occurring in new cells or existing cells may provide insight into mechanism(s) responsible for increased lifespan in CL.
To gain insight into what proportion of newly synthesized proteins are going to new cells vs. existing cellular structures, we compared the synthesis of new protein to the respective synthesis of new DNA. Comparing 4-mo CL to 4-mo Con, it appears that a greater proportion of protein synthesis is directed to cellular expansion. In contrast, in 7-mo CL mice compared with 7-mo Con, new proteins are synthesized primarily in existing cells, as illustrated by a significant increase in new proteins synthesized in relation to new DNA, indicative of new cells added to the population, across tissues. In summary, we speculate that in CL mice a tradeoff from growth to proteostasis occurs between 4 mo and 7 mo of age that is characterized by an increase in the synthesis of new protein/new DNA ratio across subcellular fractions. Therefore, maintained proteostasis may be a critical determinant in the extended lifespan and healthspan of CL mice.
CL Growth Signaling
mTORC1 is a central regulator of protein turnover, cell cycle, and mRNA translation (18). Inhibiting or downregulating mTORC1 signaling is suggested to be integral to the lifespan extension imparted by CR and chronic rapamycin treatment (14, 15, 22, 23). Expanding upon our protein synthesis findings in long-lived CL mice, we assessed two mTORC1 substrates, rpS6 and 4E-BP1, which are involved in rRNA transcription and translation initiation, respectively (7, 43). In young fasted (∼3 mo of age) mice, phosphorylation of rpS6 and 4E-BP1 was not different between CL and Con mice in any tissue, corroborating the 2-wk protein synthesis measurements in 4-mo CL. In contrast, 7-mo CL mice had greater rpS6 phosphorylation in heart and liver, whereas 4E-BP1 phosphorylation was greater in the gastroc complex compared with 7-mo Con, consistent with the increased rates of protein synthesis observed over 2 wk in CL at 7 mo. An increase in phosphorylation of mTORC1 substrates is in contrast to the reduced mTORC1 activity seen in age-matched calorie-restricted, rapamycin-treated (11, 22), and S6K1−/− mice (34) as well as the unaltered mTORC1 activity in methionine-restricted mice (39). However, branched-chain amino acid (BCAA) supplementation extends lifespan while subsequently increasing mTORC1 activity (9). Together with our current data in the CL model, this suggests that although decreasing mTORC1 activity is sufficient to extend lifespan, it may not be necessary. We propose that increases in proteostatic mechanisms may be more important factors in lifespan and healthspan than decreased mTORC1 (39).
Summary and Conclusion
Here, we demonstrate in long-lived CL mice that a transient nutrient stress during the suckling period results in long-term alterations in protein and DNA synthesis that differ between 4 and 7 mo. We propose that, compared with normal-litter-size controls, there is a greater proportion of protein synthesis directed to new cells at 4 mo, but it switches to a greater proportion of protein synthesis in existing cells, thus being directed to the maintenance of existing cellular structures, at 7 mo. We also present novel data indicating that, in CL mice, lifespan extension may be accomplished independently of reduced mTORC1 activity in primarily postmitotic tissues. These data highlight the importance of assessing pathways thought to be integral to slowed aging, such as mTORC1, as well as the metabolic outcomes, at multiple time points across the lifespan. Collectively, we expand on the body of evidence establishing CL as a novel model of longevity that provides valuable insight into protein synthesis and its role in lifespan and healthspan that could aid in the development of novel strategies to promote slowed aging.
GRANTS
This project was funded by National Institutes of Health grants 1K01 AG-O31829-01 to B. F. Miller, NIH R01 AG-042569 to B. F. Miller and K. L. Hamilton, and R01 AG-019899 to R. A. Miller.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
Author contributions: J.C.D., R.A.M., K.L.H., and B.F.M. conception and design of research; J.C.D., D.R.B., F.F.P., and L.M.B. performed experiments; J.C.D., D.R.B., F.F.P., and B.F.M. analyzed data; J.C.D., D.R.B., K.L.H., and B.F.M. interpreted results of experiments; J.C.D. prepared figures; J.C.D. drafted manuscript; J.C.D., D.R.B., R.A.M., K.L.H., and B.F.M. edited and revised manuscript; J.C.D., D.R.B., F.F.P., L.M.B., K.L.H., and B.F.M. approved final version of manuscript.
ACKNOWLEDGMENTS
We thank Kathryn Baeverstad for assistance with Western blotting and Sabrina Van Roekel for technical assistance.
REFERENCES
- 1.Austad SN. Methusaleh's zoo: how nature provides us with clues for extending human health span. J Comp Pathol 142, Suppl 1: S10–S21, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Balch WE, Morimoto RI, Dillin A, Kelly JW. Adapting proteostasis for disease intervention. Science 319: 916–919, 2008 [DOI] [PubMed] [Google Scholar]
- 3.Beltrami AP, Barlucchi L, Torella D, Baker M, Limana F, Chimenti S, Kasahara H, Rota M, Musso E, Urbanek K, Leri A, Kajstura J, Nadal-Ginard B, Anversa P. Adult cardiac stem cells are multipotent and support myocardial regeneration. Cell 114: 763–776, 2003 [DOI] [PubMed] [Google Scholar]
- 4.Bergmann O, Bhardwaj RD, Bernard S, Zdunek S, Barnabe-Heider F, Walsh S, Zupicich J, Alkass K, Buchholz BA, Druid H, Jovinge S, Frisen J. Evidence for cardiomyocyte renewal in humans. Science 324: 98–102, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Busch R, Kim YK, Neese RA, Schade-Serin V, Collins M, Awada M, Gardner JL, Beysen C, Marino ME, Misell LM, Hellerstein MK. Measurement of protein turnover rates by heavy water labeling of nonessential amino acids. Biochim Biophys Acta 1760: 730–744, 2006 [DOI] [PubMed] [Google Scholar]
- 6.Busch R, Neese RA, Awada M, Hayes GM, Hellerstein MK. Measurement of cell proliferation by heavy water labeling. Nat Protoc 2: 3045–3057, 2007 [DOI] [PubMed] [Google Scholar]
- 7.Chauvin C, Koka V, Nouschi A, Mieulet V, Hoareau-Aveilla C, Dreazen A, Cagnard N, Carpentier W, Kiss T, Meyuhas O, Pende M. Ribosomal protein S6 kinase activity controls the ribosome biogenesis transcriptional program. Oncogene 33: 474–483, 2014 [DOI] [PubMed] [Google Scholar]
- 8.Chen JH, Martin-Gronert MS, Tarry-Adkins J, Ozanne SE. Maternal protein restriction affects postnatal growth and the expression of key proteins involved in lifespan regulation in mice. PLoS One 4: e4950, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.D'Antona G, Ragni M, Cardile A, Tedesco L, Dossena M, Bruttini F, Caliaro F, Corsetti G, Bottinelli R, Carruba MO, Valerio A, Nisoli E. Branched-chain amino acid supplementation promotes survival and supports cardiac and skeletal muscle mitochondrial biogenesis in middle-aged mice. Cell Metab 12: 362–372, 2010 [DOI] [PubMed] [Google Scholar]
- 10.Dellavalle A, Sampaolesi M, Tonlorenzi R, Tagliafico E, Sacchetti B, Perani L, Innocenzi A, Galvez BG, Messina G, Morosetti R, Li S, Belicchi M, Peretti G, Chamberlain JS, Wright WE, Torrente Y, Ferrari S, Bianco P, Cossu G. Pericytes of human skeletal muscle are myogenic precursors distinct from satellite cells. Nat Cell Biol 9: 255–267, 2007 [DOI] [PubMed] [Google Scholar]
- 11.Drake JC, Peelor FF, 3rd, Biela LM, Watkins MK, Miller RA, Hamilton KL, Miller BF. Assessment of mitochondrial biogenesis and mTORC1 signaling during chronic rapamycin feeding in male and female mice. J Gerontol A Biol Sci Med Sci 68: 1493–1501, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Habbout A, Li N, Rochette L, Vergely C. Postnatal overfeeding in rodents by litter size reduction induces major short- and long-term pathophysiological consequences. J Nutr 143: 553–562, 2013 [DOI] [PubMed] [Google Scholar]
- 13.Hales CN, Barker DJ. The thrifty phenotype hypothesis. Br Med Bull 60: 5–20, 2001 [DOI] [PubMed] [Google Scholar]
- 14.Harrison DE, Strong R, Sharp ZD, Nelson JF, Astle CM, Flurkey K, Nadon NL, Wilkinson JE, Frenkel K, Carter CS, Pahor M, Javors MA, Fernandez E, Miller RA. Rapamycin fed late in life extends lifespan in genetically heterogeneous mice. Nature 460: 392–395, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hsieh CC, Papaconstantinou J. Akt/PKB and p38 MAPK signaling, translational initiation and longevity in Snell dwarf mouse livers. Mech Ageing Dev 125: 785–798, 2004 [DOI] [PubMed] [Google Scholar]
- 16.Jorgensen P, Tyers M. How cells coordinate growth and division. Curr Biol 14: R1014–1027, 2004 [DOI] [PubMed] [Google Scholar]
- 17.Kaeberlein M, Powers RW, 3rd, Steffen KK, Westman EA, Hu D, Dang N, Kerr EO, Kirkland KT, Fields S, Kennedy BK. Regulation of yeast replicative life span by TOR and Sch9 in response to nutrients. Science 310: 1193–1196, 2005 [DOI] [PubMed] [Google Scholar]
- 18.Laplante M, Sabatini DM. mTOR signaling in growth control and disease. Cell 149: 274–293, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Li G, Kohorst JJ, Zhang W, Laritsky E, Kunde-Ramamoorthy G, Baker MS, Fiorotto ML, Waterland RA. Early postnatal nutrition determines adult physical activity and energy expenditure in female mice. Diabetes 62: 2773–2783, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mary J, Vougier S, Picot CR, Perichon M, Petropoulos I, Friguet B. Enzymatic reactions involved in the repair of oxidized proteins. Exp Gerontol 39: 1117–1123, 2004 [DOI] [PubMed] [Google Scholar]
- 21.Miller BF, Hamilton KL. A perspective on the determination of mitochondrial biogenesis. Am J Physiol Endocrinol Metab 302: E496–E499, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Miller BF, Robinson MM, Bruss MD, Hellerstein M, Hamilton KL. A comprehensive assessment of mitochondrial protein synthesis and cellular proliferation with age and caloric restriction. Aging Cell 11: 150–161, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Miller BF, Robinson MM, Reuland DJ, Drake JC, Peelor FF, 3rd, Bruss MD, Hellerstein MK, Hamilton KL. Calorie restriction does not increase short-term or long-term protein synthesis. J Gerontol A Biol Sci Med Sci 68: 530–538, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Miller RA, Harrison DE, Astle CM, Baur JA, Boyd AR, de Cabo R, Fernandez E, Flurkey K, Javors MA, Nelson JF, Orihuela CJ, Pletcher S, Sharp ZD, Sinclair D, Starnes JW, Wilkinson JE, Nadon NL, Strong R. Rapamycin, but not resveratrol or simvastatin, extends life span of genetically heterogeneous mice. J Gerontol A Biol Sci Med Sci 66: 191–201, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mitchell KJ, Pannerec A, Cadot B, Parlakian A, Besson V, Gomes ER, Marazzi G, Sassoon DA. Identification and characterization of a non-satellite cell muscle resident progenitor during postnatal development. Nat Cell Biol 12: 257–266, 2010 [DOI] [PubMed] [Google Scholar]
- 26.Mortimore GE, Poso AR. Intracellular protein catabolism and its control during nutrient deprivation and supply. Annu Rev Nutr 7: 539–564, 1987 [DOI] [PubMed] [Google Scholar]
- 27.Moss FP, Leblond CP. Satellite cells as the source of nuclei in muscles of growing rats. Anat Rec 170: 421–435, 1971 [DOI] [PubMed] [Google Scholar]
- 28.Neese RA, Misell LM, Turner S, Chu A, Kim J, Cesar D, Hoh R, Antelo F, Strawford A, McCune JM, Christiansen M, Hellerstein MK. Measurement in vivo of proliferation rates of slow turnover cells by 2H2O labeling of the deoxyribose moiety of DNA. Proc Natl Acad Sci USA 99: 15345–15350, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Orlic D, Kajstura J, Chimenti S, Jakoniuk I, Anderson SM, Li B, Pickel J, McKay R, Nadal-Ginard B, Bodine DM, Leri A, Anversa P. Bone marrow cells regenerate infarcted myocardium. Nature 410: 701–705, 2001 [DOI] [PubMed] [Google Scholar]
- 30.Robida-Stubbs S, Glover-Cutter K, Lamming DW, Mizunuma M, Narasimhan SD, Neumann-Haefelin E, Sabatini DM, Blackwell TK. TOR signaling and rapamycin influence longevity by regulating SKN-1/Nrf and DAF-16/FoxO. Cell Metab 15: 713–724, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Robinson MM, Richards JC, Hickey MS, Moore DR, Phillips SM, Bell C, Miller BF. Acute β-adrenergic stimulation does not alter mitochondrial protein synthesis or markers of mitochondrial biogenesis in adult men. Am J Physiol Regul Integr Comp Physiol 298: R25–R33, 2010 [DOI] [PubMed] [Google Scholar]
- 32.Robinson MM, Turner SM, Hellerstein MK, Hamilton KL, Miller BF. Long-term synthesis rates of skeletal muscle DNA and protein are higher during aerobic training in older humans than in sedentary young subjects but are not altered by protein supplementation. FASEB J 25: 3240–3249, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sadagurski M, Landeryou T, Blandino-Rosano M, Cady G, Elghazi L, Meister D, See L, Bartke A, Bernal-Mizrachi E, Miller RA. Long-lived crowded-litter mice exhibit lasting effects on insulin sensitivity and energy homeostasis. Am J Physiol Endocrinol Metab 306: E1305–E1314, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Selman C, Tullet JM, Wieser D, Irvine E, Lingard SJ, Choudhury AI, Claret M, Al-Qassab H, Carmignac D, Ramadani F, Woods A, Robinson IC, Schuster E, Batterham RL, Kozma SC, Thomas G, Carling D, Okkenhaug K, Thornton JM, Partridge L, Gems D, Withers DJ. Ribosomal protein S6 kinase 1 signaling regulates mammalian life span. Science 326: 140–144, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Senyo SE, Steinhauser ML, Pizzimenti CL, Yang VK, Cai L, Wang M, Wu TD, Guerquin-Kern JL, Lechene CP, Lee RT. Mammalian heart renewal by pre-existing cardiomyocytes. Nature 493: 433–436, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Stadtman ER. Protein modification in aging. J Gerontol 43: B112–120, 1988 [DOI] [PubMed] [Google Scholar]
- 37.Stadtman ER. Protein oxidation and aging. Science 257: 1220–1224, 1992 [DOI] [PubMed] [Google Scholar]
- 38.Steinbaugh MJ, Sun LY, Bartke A, Miller RA. Activation of genes involved in xenobiotic metabolism is a shared signature of mouse models with extended lifespan. Am J Physiol Endocrinol Metab 303: E488–E495, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Sun L, Sadighi Akha AA, Miller RA, Harper JM. Life-span extension in mice by preweaning food restriction and by methionine restriction in middle age. J Gerontol A Biol Sci Med Sci 64: 711–722, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Tarry-Adkins JL, Martin-Gronert MS, Chen JH, Cripps RL, Ozanne SE. Maternal diet influences DNA damage, aortic telomere length, oxidative stress, and antioxidant defense capacity in rats. FASEB J 22: 2037–2044, 2008 [DOI] [PubMed] [Google Scholar]
- 41.Verdijk LB, Koopman R, Schaart G, Meijer K, Savelberg HH, van Loon LJ. Satellite cell content is specifically reduced in type II skeletal muscle fibers in the elderly. Am J Physiol Endocrinol Metab 292: E151–E157, 2007 [DOI] [PubMed] [Google Scholar]
- 42.Walsh S, Ponten A, Fleischmann BK, Jovinge S. Cardiomyocyte cell cycle control and growth estimation in vivo—an analysis based on cardiomyocyte nuclei. Cardiovasc Res 86: 365–373, 2010 [DOI] [PubMed] [Google Scholar]
- 43.Wang X, Beugnet A, Murakami M, Yamanaka S, Proud CG. Distinct signaling events downstream of mTOR cooperate to mediate the effects of amino acids and insulin on initiation factor 4E-binding proteins. Mol Cell Biol 25: 2558–2572, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.White RB, Bierinx AS, Gnocchi VF, Zammit PS. Dynamics of muscle fibre growth during postnatal mouse development. BMC Dev Biol 10: 21, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Wilkinson JE, Burmeister L, Brooks SV, Chan CC, Friedline S, Harrison DE, Hejtmancik JF, Nadon N, Strong R, Wood LK, Woodward MA, Miller RA. Rapamycin slows aging in mice. Aging Cell 11: 675–682, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Wu P, Jiang C, Shen Q, Hu Y. Systematic gene expression profile of hypothalamus in calorie-restricted mice implicates the involvement of mTOR signaling in neuroprotective activity. Mech Ageing Dev 130: 602–610, 2009 [DOI] [PubMed] [Google Scholar]
- 47.Zheng B, Cao B, Crisan M, Sun B, Li G, Logar A, Yap S, Pollett JB, Drowley L, Cassino T, Gharaibeh B, Deasy BM, Huard J, Peault B. Prospective identification of myogenic endothelial cells in human skeletal muscle. Nat Biotechnol 25: 1025–1034, 2007 [DOI] [PubMed] [Google Scholar]