Fig. 6.
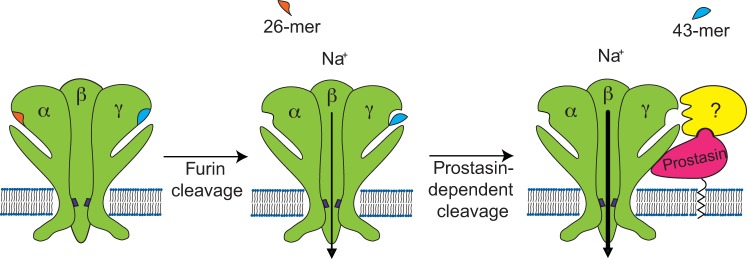
Hypothetical mechanism of activation of ENaC by serine proteases. ENaC α- and γ-subunits contain embedded inhibitory tracts in their extracellular loops. ENaC subunits are assembled in a trimeric structure in the biosynthetic pathway. During maturation in the trans-Golgi network, the pro-protein convertase furin cleaves the α-subunit twice, releasing a 26-mer inhibitory tract, and the γ-subunit at a single site preceding the γ inhibitory tract. Channels lacking proteolytic cleavage by furin have a very low open probability (Po) in the presence of external Na+. Channels that have been processed by furin and therefore lack the α-subunit inhibitory tract have an intermediate Po of ∼0.3–0.4. Prostasin is associated with the plasma membrane through a glycosylphosphatidylinositol anchor and interacts with ENaC. We propose that prostasin can cleave ENaC as well as recruit other serine proteases to the ENaC-prostasin complex, which eventually can cleave the γ-subunit, releasing the inhibitory peptide. The release of the inhibitory peptide from the γ-subunit promotes a further increase in Po. Channels lacking the inhibitory tract in both the α- and γ-subunits have a Po that approaches 1.
