Abstract
Inhomogeneous inflation or deflation of the lungs can cause dynamic pressure differences between regions and lead to interregional airflows known as pendelluft. This work first uses analytical tools to clarify the theoretical limits of pendelluft at a single bifurcation. It then explores the global and regional pendelluft that may occur throughout the bronchial tree in a realistic example using an in silico model of bronchoconstriction. The theoretical limits of pendelluft volume exchanged at a local bifurcation driven by sinusoidal breathing range from 15.5% to 41.4% depending on the relative stiffness of the subtended regions. When nonsinusoidal flows are considered, pendelluft can be as high as 200% inlet tidal volume (Vin). At frequencies greater than 10 Hz, the inertia of the air in the airways becomes important, and the maximal local pendelluft is theoretically unbounded, even with sinusoidal breathing. In a single illustrative numerical simulation of bronchoconstriction with homogenous compliances, the overall magnitude of global pendelluft volume was <2% of the tidal volume. Despite the small overall magnitude, pendelluft volume exchange was concentrated in poorly ventilated regions of the lung, including local pendelluft at bifurcations of up to 13% Vin. This example suggests that pendelluft may be an important phenomena contributing to regional gas exchange, irreversible mixing, and aerosol deposition patterns inside poorly ventilated regions of the lung. The analytical results support the concept that pendelluft may be more prominent in diseases with significant heterogeneity in both resistance and compliance.
Keywords: ventilation distribution, ventilation mechanics, gas exchange, asthma, computational modeling
inspiratory and expiratory airflows in healthy lungs are relatively uniform throughout the bronchial tree. However, in pulmonary diseases or under abnormal conditions, this uniform pattern can be disturbed; inhomogeneous inflation or deflation of the lungs can cause dynamic pressure differences between different regions, which in turn lead to interregional airflows. This effect is referred to as pendelluft (“swinging air”) because gas is passed back and forth between the different regions of the lungs.
Pendelluft occurs when regions of the lung have different dynamics of regional inflation and deflation. For example, in a lung with two regions of equal compliance, inspiratory airflow is diverted away from a region with higher resistance (R), and incomplete equilibration leads to lower end-inspiratory pressure in the region with higher resistance compared with the other region. If the inspiration is followed by a pause, having zero flow at the airway opening, the pressure difference will cause pendelluft flow from one region to the other until the pressures are equilibrated. If the region with the higher resistance also has a greater compliance (i.e., it is less stiff) than the other region, more pendelluft flow will be needed to balance the pressures throughout the lungs. Experimental evidence and theoretical aspects of pendelluft attributable to varied resistance and compliance were first described in a classic paper by Otis et al. in 1956 (12), which included an excellent analysis of frequency dependence of resistance and compliance at a bifurcation as well as a theoretical upper limit for pendelluft.
Clinically, pendelluft can be observed during mechanical ventilation of patients with unilateral chest or lung injury, where the two lungs can sometimes be seen inflating and deflating out of phase with each other (6, 16). More subtle pendelluft is sometimes observed as a gradual drop in pressure during an end-inspiratory pause, which may be caused by airflow between the different regions of the lungs. There is emerging evidence of pendelluft in bronchoconstricted asthmatics (15), in subjects with chronic obstructive pulmonary disease (20), and during mechanical ventilation under certain conditions including spontaneous effort (22).
At breathing frequencies much higher than those at rest, differences in how air mass within parallel regions of the lungs respond to rapid pressure changes (characterized by their inertance, L) can also lead to pendelluft. This kind of pendelluft has been postulated to aid gas exchange during high-frequency ventilation (7, 8, 18). Although this effect was not explicitly included in the analytical description of pendelluft by Otis et al. (12), their mathematical model would allow adding inertive terms. Other investigators included inertance in more complicated analytical descriptions (7, 18), and in a sophisticated model of airflows in an airway tree (1).
Since the 1956 paper of Otis et al. (12), work on pendelluft at a single bifurcation has continued analytically, numerically, and experimentally. For example, pendelluft can affect gas transport by displacing air in the anatomical dead space within the volume of the airways (14). It has also been shown that pendelluft can be observed even in symmetrical bifurcations if instabilities (like those that emerge from nonlinearities in airway resistance) are considered (9). Additionally, pendelluft may be an important physiological phenomenon for irreversible particle and gas mixing in the bronchial tree (3, 17).
Pendelluft in a multibranching bronchial tree is more complicated than that at a single bifurcation, as it may occur at several bifurcations. In particular, pendelluft that occurs at multiple airway bifurcations between the central airway and the terminal units results in some form of stacking or propagation of the effects of pendelluft across airway generations. Pendelluft throughout the tree increases the overall tidal expansion of the acini, albeit with an uncertain combination of fresh gas and gas that has already been resident elsewhere in the lungs. We reasoned that this additional pendelluft volume could be explored using a numerical model of the airway tree during bronchoconstriction (19). This model would thus permit quantitative exploration of the magnitude and effect of pendelluft within a bronchial tree in an illustrative example.
This study has two distinct aims that are mutually supportive. The first is to clarify the definition, causes, and magnitude of pendelluft. To this end, we use analytical tools to define a generalized quantitative definition of pendelluft, identify its limits, and evaluate its magnitude using analytical methods. We then extend this definition to include pendelluft throughout a bronchial tree. The second aim of the paper is to provide an example of local and regional effects of pendelluft on airflow and ventilation in a realistic context using an in silico model of bronchoconstriction. We then use the numerical simulation to explore the frequency dependence of global pendelluft volume and the conditions for which the inertance of the airways becomes important.
METHODS
Pendelluft flow, volume, and ventilation definition.
Pendelluft flow, volume, and ventilation definition at a bifurcation are based on an earlier definition by Otis et al. (12). Despite the complicated dynamics of airflows during pendelluft, Otis et al. (12) found an analytical description of the pendelluft volume at a bifurcation over a breathing period by using a circuit model of the lungs including resistances and compliances (Fig. 1). Otis defined the relative pendelluft volume at a given bifurcation as the excess in the sum of the tidal volumes of two daughter airways relative to the tidal volume delivered through the parent airway. This pendelluft volume exchanged between regions increases the sum of the daughter tidal volumes without affecting the parent tidal volumes. This ratio quantifies pendelluft at a bifurcation.
Fig. 1.
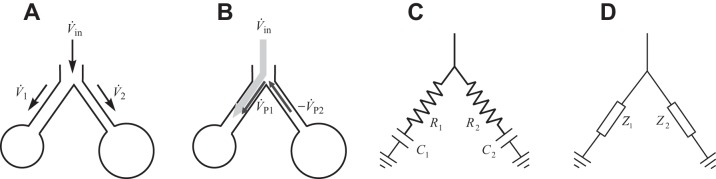
Schematics of single bifurcation models to describe pendelluft. A: definition of local parent flow and daughter flows. B: example of a pendelluft flow condition at the beginning of inspiration, note the pendelluft flow going from one daughter into the other, combining with parent flow here in the left branch. Flows in the 2 daughters have opposite signs. C: resistance-compliance model of a bifurcation. D: general complex impedance model of a bifurcation. For definitions of abbreviations, please refer to the Glossary.
We seek to extend this definition to include pendelluft flow over time, understand its limitations, and to ultimately use it to characterize the intergenerational aggregation of pendelluft throughout the global bronchial tree. However, before quantifying this aggregation, it is helpful to first identify the features that characterize pendelluft flow originating at an individual bifurcation. 1) Pendelluft flow occurs if and only if the flows in the daughter branches have opposite sign (Fig. 1B). 2) Pendelluft flow passes from one daughter to the next and is therefore equal in magnitude in both daughters. 3) This magnitude is equal to the smaller flow of the two daughters, which flows in the opposite direction as the parent and is entirely pendelluft. If there is no flow through the parent, then both daughter flows are equal in magnitude, and both are entirely pendelluft. 4) The direction of the pendelluft flow in each of the daughter airways is the same as the direction of the total flow in the airway. These observations lead to the following definition of pendelluft flow emerging at an individual bifurcation:
| (1) |
The subscript j indicates either of the two daughter airways in a bifurcation (j = 1,2 for all subscripts in this paper). V̇1, V̇2 are the overall flows, and V̇P,1, V̇P,2 are the pendelluft flows. The total bifurcation pendelluft flow V̇PB is the sum of the magnitude of the daughter pendelluft flows and is signed (arbitrarily) with V̇1:
| (2) |
An equivalent definition to the one presented in Eqs. 1 and 2, but without the piecewise character of Eq. 1 and the minimum function is:
| (3) |
where V̇in is the parent flow, which by conservation of mass satisfies V̇in = V̇1 + V̇2. Integration of the absolute amount of pendelluft flow over the breathing period T yields the local pendelluft volume at a bifurcation:
| (4) |
where the volumes VPB, V1, V2, Vin are defined as:
| (5) |
for any subscript x.
Note that Vin at the carina is the conventional tidal volume VT of the whole lung, and the factor of ½ converts the integral of the absolute airflow over the breathing cycle to VT; this is equivalent to an integral over inspiration or expiration only, provided there are no breath-to-breath changes in end-expiratory lung volume, which would require an average over multiple breathing cycles. The relative pendelluft volume at a bifurcation is defined as the pendelluft volume normalized by the local tidal volume delivered through the parent airway, which matches the description of Otis et al. (12):
| (6) |
Pendelluft can occur at many bifurcations throughout a given region of the bronchial tree, and its aggregation over the generations of the airway tree determines the overall increase in ventilation of the region. The regional pendelluft volume (denoted VP) is normalized to the tidal volume feeding the region (denoted Vin) to yield the normalized regional pendelluft volume VP/Vin. For the set of bifurcations β within the tree or subtree, and the set of terminal airways of the tree or subtree Τ:
| (7) |
Note that when the set includes the entire tree, VP/Vin is the global pendelluft. Also, note that the regional pendelluft is evident from the sum of tidal volumes of the terminal airways compared with the inlet tidal volume. As proof of this, consider a tree with two generations and three bifurcations. Summing the pendelluft of these bifurcations (each given by Eq. 4) results in an algebraic combination of the airway tidal volumes that simplifies to the sum of tidal volumes of terminal units minus the inlet tidal volume. Normalizing by the inlet tidal volume gives the result shown in Eq. 7. Note the terminal airways used in Eq. 7 are those at the end of any defined portion of the airway tree with a single inlet and need not be those airways that directly feed the compliances. Therefore, the regional relative pendelluft definition in Eq. 7 reduces to the bifurcation definition in Eq. 6 because a single bifurcation may be considered as a subtree with two terminal airways.
While the regional pendelluft volume is a quantification including all local pendelluft volumes in a region, it does not show how the local pendelluft generated at bifurcations propagates through the airway tree. To quantify how pendelluft is distributed throughout the bronchial tree, we define the aggregate pendelluft volume VAP,j as the volume through the jth daughter airway of a given bifurcation, which has accumulated from pendelluft in that or more proximal bifurcations. This volume combines with bulk flow VB,j that has never experienced pendelluft in the total flow through the airway Vj:
| (8) |
Recall that the subscript j indicates either of the two daughter airways in a bifurcation (j = 1,2 for all subscripts in this study).
The aggregate pendelluft itself is a combination of the local pendelluft VP,j and a fraction of the aggregate pendelluft of the parent airway VAP,in. Because the daughter airways cannot distinguish bulk volume from aggregate pendelluft volume, this fraction is the same as the fraction of ventilation that goes to that airway.
| (9) |
Note that the earlier definition of local and regional pendelluft considered pendelluft that emerges in a bifurcation or subtree, without considering pendelluft from more proximal bifurcations in the bronchial tree that can arrive through the parent airway. In contrast, the aggregate pendelluft deals with this issue directly and considers the total pendelluft driven volume that passes through a given airway.
This concludes our definitions of pendelluft flow and the volume of local, regional, and aggregate pendelluft. We next use analytical methods to evaluate the magnitude of pendelluft volume at a single bifurcation.
Analytical characterization of pendelluft.
Analytical characterization of pendelluft in a model with linear elements can be illustrated using the electrical analogs of resistance and compliance (Fig. 1C) or more generally with impedance elements (Fig. 1D). The components represent two daughter branches and subtended regions at any bifurcation in the tree. The amplitude of a sinusoidal flow through an impedance is given by the amplitude of the driving pressure divided by the magnitude of the impedance.
When such a system is driven with a single sinusoidal frequency the pendelluft volume over a breathing period can be expressed directly in terms of these impedances:
| (10) |
where Z1 and Z2 are the complex impedances of the daughters (Fig. 1D).
This equation is equivalent to the one found originally by Otis et al. (12). It is limited to a single bifurcation driven with a single frequency and is evaluated over a complete period. Note that the impedances are complex, and the absolute value operator is that defined for complex numbers. Given that the initial definition of pendelluft flow (Eq. 1) includes a piecewise definition, a minimum function, and absolute value operators, it is remarkable that the pendelluft volume of the breathing cycle can be expressed in this simpler form. This form permits calculations in networks of impedances such as the airway tree. However, note that Eq. 10 remains nonanalytical because of the absolute value operators; meaning that it is not additive, and harmonic decomposition cannot be used to evaluate nonsinusoidal driving pressures.
Equation 10 can be derived directly from Eq. 4, but some observations simplify this considerably. The local pendelluft volumes can be evaluated using only the amplitudes of the input flow and the flow through the daughters without directly considering the phase difference between the flows. This is because integrating over a complete cycle to identify the tidal volume of each of the daughters' subtended regions is independent of the phase difference (even though the phase difference between the daughters is important, indeed key to the existence of pendelluft). These subtended volumes are determined by the magnitude of the daughter impedances, and, after normalizing to the input flow, Eq. 10 is reached. See also the geometric construction in Fig. 4.
Fig. 4.
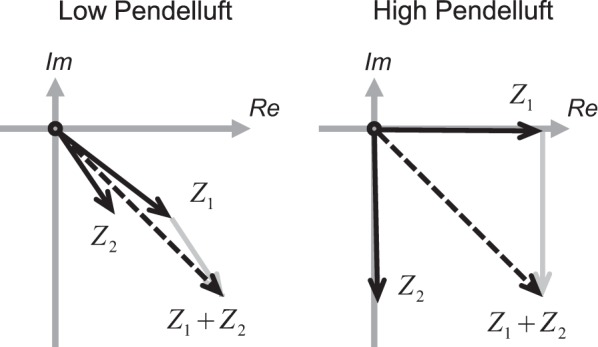
A geometrical description of the combinations of daughter impedances (Z1 and Z2) that lead to low and high pendelluft. For RC models of the lung, these vectors are confined to the lower right quadrant of the complex plane. Higher pendelluft happens when the impedances diverge and the sum of the daughter magnitudes is considerably larger than the magnitude of their sum.
The pendelluft equation (Eq. 10), although only valid at a single sinusoidal frequency, permits any linear element to be included in the circuit. This can include conditions at very high breathing frequencies, where the effect of inertance on airflows and pressures becomes important. At normal breathing frequencies, however, the airway tree can be modeled as a combination of resistances and compliances. Otis et al. (12) pointed out that any bifurcation within such a tree might be modeled using a single frequency-dependent effective resistance and compliance for each daughter branch. We therefore expressed Eq. 10 explicitly for any RC circuit in Eq. 11 and in doing so describe pendelluft volume at any bifurcation of the airway tree during normal breathing. The resulting equations were used to find the analytical limits of relative pendelluft for a range of circuits and conditions (Eqs. 12–14).
In the next section, we describe the methodology of the second aim in this paper: to use an in silico model of bronchoconstriction to illustrate both local and global effects of pendelluft on airflow and ventilation in a realistic context and nonsinusoidal input.
Numerical simulation of pendelluft.
Numerical simulation of pendelluft was used to illustrate the pendelluft that can occur during heterogeneous bronchoconstriction observed in asthma. An integrative model of bronchoconstriction including an airway tree with 12 generations was used to simulate a pattern of the self-organized airway constriction that emerges during an asthma attack, as previously described in detail (19). Briefly, our computational model involves solving the distribution of airflow, pressure, and volume within a bronchial tree with 12 generations of branching using Euler's method for numerical integration and time steps of 10 ms. The dynamics of airflow distribution in the model are determined by the input at the central airway opening and recursive equations for the network of resistances connected to the compliances of the terminal units. Airway radii were updated breath by breath according to the relative airway smooth muscle tone (Tr) and the peak transmural pressure of the airway during the breathing cycle, taking into account the transmural pressure and parenchymal forces. The simulation using Tr = 90%, a mechanical ventilation profile with a volume-controlled mode, constant inspiratory flow, a tidal volume of 650 ml, positive end-expiratory pressure of 5 cmH2O, and 12 breaths per minute for a period of 600 breaths resulted in the emergence a typical steady-state pattern of heterogeneous airway constriction (Fig. 2) (19). This pattern of bronchoconstriction within the airway tree is consistent with the emergence of ventilation defects (regions of gas trapping or very poor ventilation) in asthma (21). The fraction of closed or hypoventilated terminal units receiving less than 15% of the average ventilation was 16.7%, indicating substantial ventilation defects. We used that pattern of heterogeneous airway constriction including the 8,191 airways of the model and simulated the airflows in all of the airways of the model over a complete breathing cycle. These airflows were used to calculate the pendelluft flow and volumes using Eqs. 3 and 4, respectively.
Fig. 2.
Example of self-organized bronchoconstriction used to explore pendelluft in a realistic context. Note that the constricted airways group together, leading to regionally clustered ventilation defects, and that there are substantial differences in constriction among daughter airways. The airways are shown as points and the connectivity among the airways as lines; darker areas illustrate higher local density.
To explore the effect of breathing waveform on pendelluft, we used the pattern of airway constriction and applied a sinusoidal breathing pattern. The airflows throughout the tree were used to calculate local and global pendelluft volumes. An example of pendelluft flow at a bifurcation is presented in Fig. 6 for both the sinusoid and mechanical ventilation breathing simulations. In addition, the global relative pendelluft volume of the bronchial tree, the maximum and average local relative pendelluft volume at a bifurcation, the average fraction of the breathing cycle a bifurcation exhibited pendelluft flow, and the fraction of the breathing cycle that pendelluft occurred somewhere in the bronchial tree were tabulated. Finally, the frequency dependence of global pendelluft volume in the numerical example was explored using this same steady-state solution for the airway diameters.
Fig. 6.
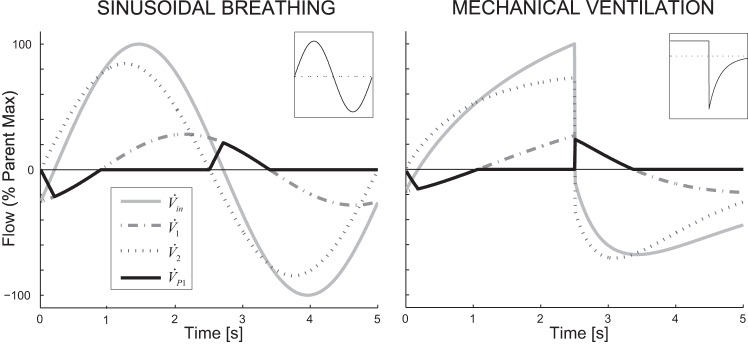
Example of flows at the bifurcation with the highest pendelluft volume (both are at generation 10). Inset: flow profile into the airway tree. The flows within the daughters sum to the parent flow. Pendelluft flow begins and ends when one of the flows crosses 0 and changes sign.
RESULTS
Theoretical limits for pendelluft.
Theoretical limits for pendelluft with sinusoidal airflow and at a single bifurcation were derived from the analytical evaluation of Eq. 10. Despite the five parameters in the physical circuit (two resistances, two compliances, and the frequency) shown in Fig. 1C, the pendelluft depends only on three independent parameters and κ:
| (11) |
where and are the time constants, nondimensionalized by frequency, = ωτ1, τ1 = R1C1; = ωτ2, τ2 = R2C2; and κ = C1/C2. Importantly, note that and can also be read as the frequency, nondimensionalized by the respective time constants. This function is explored in Fig. 3 for κ = 1. As κ increases from unity, the maximum on the horizontal axis increases and moves to the right.
Fig. 3.
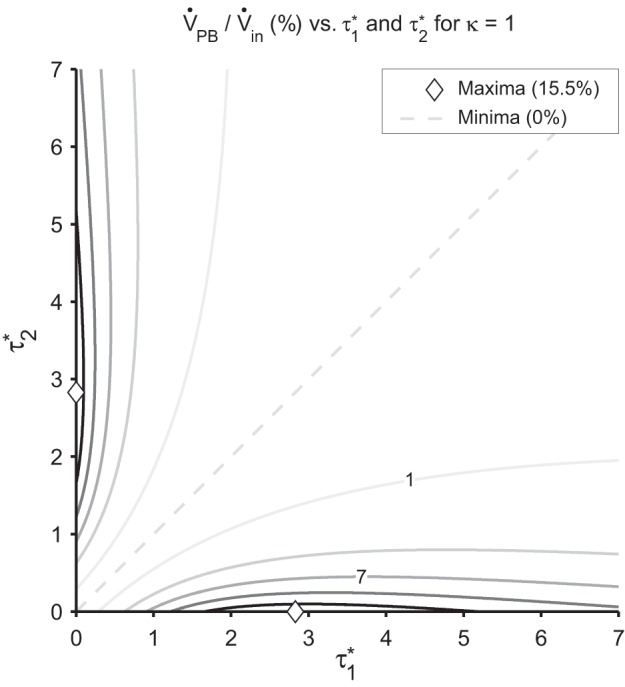
Isocontours marking 3% increments in local relative pendelluft volume as a function of the nondimensional time constants, for κ = 1. The shape of the isocontours shows the steep transition from the maximum at the ◇ (15.5%) to the minimum (0) on the line of symmetry.
The impedances in Eq. 10 can be represented with vectors in the complex plane. The specific elements in the circuit determine the quadrant(s) of this plane from which the daughter impedances can be chosen. A circuit without inertance (i.e., an RC circuit) can only have impedances in the lower right quadrant, whereas a circuit without compliance effects (i.e., an RL circuit) can only have impedances in the upper right quadrant. When all effects are considered (i.e., an RLC circuit) the daughter impedances can be anywhere in the right half of the complex plane. Equation 10 is maximized when the sum of the impedance magnitudes are large and when the magnitude of the sum of the impedances are small (i.e., the vectors tend to cancel each other out). Geometrical proofs that find the combination of impedance vectors that maximize pendelluft (similar to what is illustrated in Fig. 4) result in the following limits for relative pendelluft volume at a bifurcation.
First, for an RC circuit with a fixed ratio of terminal compliances κ = C1/C2, where C1 ≥ C2, the maximum pendelluft is achieved when =[(κ + 1)(κ + 3)]1/2 and = 0. At this point we have:
| (12) |
The relationship between the maximum relative pendelluft volume and κ is plotted in Fig. 5. The maximum with respect to for any fixed κ occurs when one daughter has vanishingly low compliance or resistance → 0). These maxima over increase with κ and have an asymptotic limit as κ → ∞, given by:
| (13) |
Fig. 5.
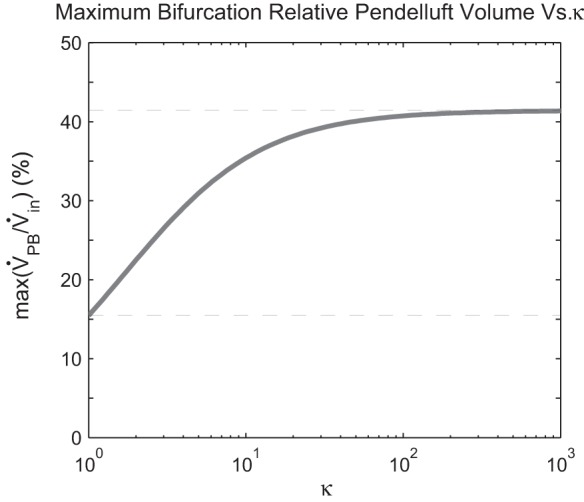
The maximum possible relative pendelluft varies with the ratio of the effective compliances of the 2 daughter pathways. A bifurcation with matched stiffness (κ = 1) gives the lower limit, whereas the upper limit is reached when the path with lower resistance (always subscript 1) has a higher stiffness.
This overall maximum matches Otis et al. (12) and is approached as one daughter resistance decreases and the other daughter compliance increases, such that the magnitudes of the impedances in the two branches are equal (as in Fig. 4, high pendelluft). In this case = 1 (R1 = 1/C2ω), → 0, and κ → ∞. If the compliances are equal (κ = 1), the maximum over is:
| (14) |
In this case → 0 and = 2√2. This 15.5% maximum is different than the 5.5% maximum presented in Otis et al. (12), but, as the fundamental formulas in that paper are correct, this is most likely a typographical error in Otis et al. (12), rather than an error of substance.
Second, RL circuits with resistance and inertance (without capacitance) are not typically relevant for the bronchial tree but can give insight into the conditions that lead to pendelluft in circuits during high-frequency ventilation. These circuits have the same limits as RC circuits presented in the equations and figures above, where the dimensionless variables are redefined as = R1/L1ω, = R2/L2ω, and κ = L2/L1. Maxima are found when the daughter with high inertance has low resistance.
Third, RLC circuits with resistances, inductances, and capacitances can have unbounded pendelluft. In these cases, pendelluft may oscillate back and forth between the daughters if the resistances are sufficiently small that the system is underdamped. The characteristic frequency as R1 + R2 → 0 is given by ω2 = [1/(L1 + L2)] * [(C1 + C2)/(C1C2)] (series inductance, series capacitance).
The limits presented above apply, like the results in the Otis paper (12), only to pressure or flow-driven inputs at a single frequency. For other waveforms, the pendelluft volume may exceed these values. For example, consider a circuit comprising vanishing resistance in one branch and unbounded compliance in the other (the case above, for which relative pendelluft may reach 41% if driven at the appropriate single frequency). Driving this with a step change in pressure would instantaneously fill the compliance with the tidal volume followed by the discharge of the compliance through the resistor. During the instantaneous filling, no volume is passed through the resistive branch. Subsequently, if an exponential pressure drop matching the pressure inside the compliance is applied to the parent, the capacitance will discharge entirely through the parallel resistance. Reversing the input pressure would reverse the process. Here the normalized tidal volumes for the compliance daughter, resistance daughter, and the whole circuit are 2, 1, and 1, respectively. On the basis of Eq. 6, this yields a relative pendelluft volume of 200%. In contrast, we will shortly show that other nonsinusoidal flows such as the one used in the mechanical ventilation simulation can lead to lower pendelluft than a sinusoidal flow at the same frequency.
Illustrative example of pendelluft in the bronchial tree.
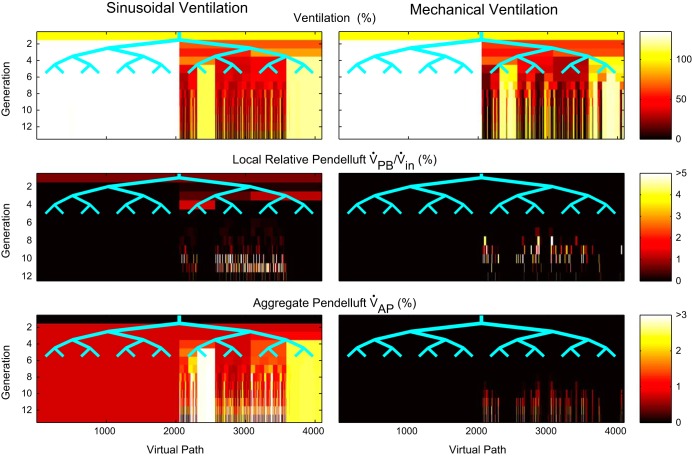
An illustrative example of pendelluft in the bronchial tree was explored using numerical simulations. Both sinusoidal and mechanical ventilation profiles were applied to a simulation of self-organized bronchoconstriction in an airway tree. Pendelluft flow was observed in both the sinusoidal and mechanical profiles at bifurcations within poorly ventilated regions of the lung (Fig. 6). Note that the originally constant flow inhalation profile applied to the airway tree during mechanical ventilation is changed to the input ventilation profile of the bifurcation by the frequency-dependent dynamics of the airway tree. Table 1 shows metrics of pendelluft for the two simulations.
Table 1.
Pendelluft simulation results for the two simulations
| Sinusoidal Ventilation | Mechanical Ventilation | |
|---|---|---|
| Global relative pendelluft volume, % VT | 1.57 | 0.13 |
| Maximum local relative pendelluft volume, % Vin | 12.67 | 13.45 |
| Average local relative pendelluft volume, % Vin | 0.25 | 0.10 |
| Average fraction of breathing cycle in local pendelluft, % Total | 3.12 | 1.80 |
| Fraction of breathing cycle with any pendelluft, % Total | 78.8 | 100 |
See Glossary for definition of terms.
Figure 7 shows the distribution of ventilation, relative pendelluft, and aggregate pendelluft volumes throughout the bronchial tree for the sinusoidal and ventilator waveforms. The distribution at each generation of the tree is visualized with a number of boxes matching the number of airways at that generation. The color profile along a vertical line from the top to the bottom represents theoretical streamtubes that go from the most central airway generation of the model to the terminal airways like a bundle of virtual paths. The Fig. 7, top, shows the ventilation pattern during steady-state bronchoconstriction for each waveform as a percent of the ventilation the airway would experience in a perfectly symmetric tree (% Uniform Vin). Using this normalization allows us to see the smaller volumes in the higher generations. The dark regions indicate poorly ventilated regions that are similar for the two ventilation profiles. Figure 7, middle, shows the magnitude of relative pendelluft volume at each bifurcation. Observe that regions of high relative pendelluft volume (yellow and red lines) are almost always within the ventilation defects in Fig. 7, top. This is where substantial differences in constriction between neighboring pathways exist (Fig. 2). Also, there was pendelluft in more central airways for sinusoidal waveform (dark red areas) but not for mechanical ventilation. Figure 7, bottom, shows the distribution and buildup of aggregate pendelluft volume (% Uniform Vin). These panels illustrate how pendelluft generated at a bifurcation is redirected to better ventilated regions within the ventilation defect.
Fig. 7.
Top: bronchoconstricted ventilation pattern with darkly colored regions of reduced ventilation as a percent Uniform Vin. Middle: high relative pendelluft emerging within ventilation defects is shown with bright colors. Bottom: regional aggregate pendelluft volume illustrating the diversion to better ventilated regions as a percent Uniform Vin.
The frequency dependence of global pendelluft volume in the bronchial tree as a function of the frequency of the driving signal is shown in Fig. 8. The maximum total global pendelluft volume for a single frequency sinusoid was 1.75% of the input volume (VT) at 0.02 Hz. Gas inertia becomes important above about 10 Hz and creates the second peak with a maximum of 1% at 400 Hz.
Fig. 8.
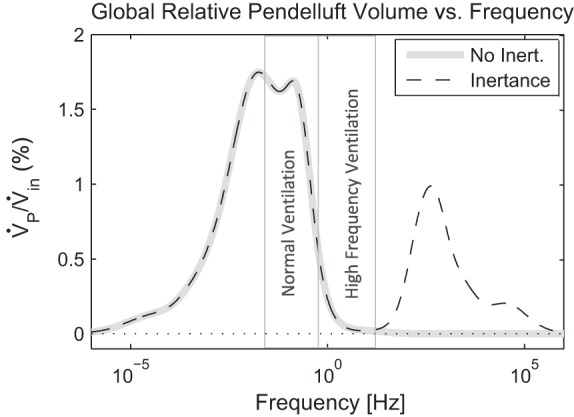
Frequency dependence of global pendelluft volume in a bronchial tree for the example of heterogeneous bronchoconstriction showing the effect of sinusoidal input at different frequencies. Both models of the tree that exclude (No Inert.) or include (Inertance) the effects of the air mass are shown. The physiologically relevant ranges for normal and high-frequency ventilation are indicated with vertical gray lines.
DISCUSSION
Key findings.
The key findings in this work can be separated into analytical and numerical results. In the first portion of this work, we clarified the definition of pendelluft and generalized this definition to include nonsinusoidal flows as well as to a multigenerational tree. This allowed us to explore local pendelluft at bifurcations, aggregate pendelluft volume that builds up over the bronchial tree, and the global pendelluft volume over the entire tree. We then used analytical tools to identify the limits of pendelluft at a bifurcation for a range of airway models. For a conventional resistance and compliance model of the airway tree driven by a single frequency sinusoid, we found that the limits of possible pendelluft depend on the ratio of compliances of the daughter pathways. When these compliances were equal, the maximum possible pendelluft volume was 15.5% of the tidal volume of the bifurcation. [As noted in results, this is different from the 5.5% presented by Otis et al. (12), which is most likely a typographical error]. When these compliances are dissimilar, the maximum pendelluft for a sinusoidal breathing pattern rises to 41%. The difference in the upper limit of pendelluft between the two conditions suggests that pendelluft may be higher in diseases causing heterogeneous changes in compliance such as emphysema. When the effects of inertance are also considered, it is theoretically possible to have any amount of pendelluft. We also introduced a nonsinusoidal waveform that could result in pendelluft volumes that were twice the tidal volume of the bifurcation, even without inertive effects.
In the second portion of this work, an illustrative example of pendelluft in the bronchial tree was explored using numerical simulations. Both sinusoid and typical mechanical ventilation profiles were applied to an instance of self-organized bronchoconstriction in an airway tree. The main quantitative findings were as follows: 1) a small amount of pendelluft occurs at bifurcations in a realistic model of bronchoconstriction; 2) local pendelluft can be as high as 13% relative to tidal volume flowing through the parent airway and is most commonly found in poorly ventilated regions of the lung attributable to the increased heterogeneity between parallel pathways (Fig. 2) that occurs within ventilation defects during bronchoconstriction (21); and 3) pendelluft volume created at any given bifurcation level is distributed to the better ventilated distal regions.
Lastly, we characterized the frequency dependence of pendelluft in the example and found two separate regions where global pendelluft emerged. The first region happens at lower frequencies and is driven by heterogeneous resistance and compliance. The maximum for this type of pendelluft was <2% of the tidal volume and occurs at near normal breathing frequencies. Above 10 Hz, a second mode of pendelluft emerges for models that include inertance. The maximum for this kind of pendelluft was 1% of the tidal volume and emerged at frequencies much larger than those used for high-frequency ventilation.
Sinusoidal input resulted in greater pendelluft than mechanical ventilation (Fig. 7, middle). The particular frequency chosen for the simulations (12 breaths/min) may have also contributed to higher pendelluft with the sinusoidal waveform because the frequency dependence of global pendelluft volume (Fig. 8) showed a maximum near 12 breaths/min. Pendelluft occurred somewhere in the tree during 78.8% (sinusoidal input) or 100% (mechanical ventilation) of the breathing cycle despite its small overall magnitude.
Contribution of pendelluft to overall gas transport.
The contribution of pendelluft to overall gas transport depends on its magnitude and the composition of the added pendelluft volume. In the bronchoconstriction example, the magnitude of the global pendelluft volume was <2% relative to the tidal volume that passed through the central airways. To determine the gas composition of pendelluft flow throughout the airway tree would require modeling the gas transport through the actual volume of the airways for dynamic flow conditions. For example, the gas composition of pendelluft flow at one point of the airway tree could be fresh gas that would have otherwise ended up in the anatomical deadspace, or it could be gas coming from neighboring alveolar units. The small magnitude of the pendelluft volume suggests that, even if it were fresh gas, it is unlikely to be an important phenomenon for overall gas exchange. On the other hand, pendelluft that occurs at bifurcations within ventilation defects (Fig. 7) may be important for gas exchange if it could, for example, increase oxygen delivery in hypoventilated terminal units.
Comparisons with experimental studies.
Comparisons with experimental studies are limited because it is challenging to experimentally approximate the flow patterns throughout the bronchial tree; our example of bronchoconstriction in asthma clearly showed the complexity of local pendelluft at the distal bifurcations of the bronchial tree and its propagation throughout the generations of the tree. However, comparison with experimental data focusing on the larger airways and proximal bifurcations is possible. Shinozuka et al. (16) measured pendelluft at the carina in an animal model of flail chest. They measured the magnitude of pendelluft volume transferred between the two lungs to be <2% of the tidal volume. In flail chest, we expect the collapsed lung to be both stiffer (as the chest wall is no longer supporting the lung) and have higher resistance (attributable to reduced size of the lung). Pendelluft is highest when the stiffer lung has lower resistance, and the findings of a small local pendelluft volume at the carina in flail chest are consistent with the results presented here.
In another experimental study of pendelluft in dogs with flail chest, Harada et. al. (6) found pendelluft volumes exchanged by one of the daughters to be as high as 12.5% of the total volume passing through that branch. This is similar in magnitude to the maximum relative pendelluft observed in the simulations at low frequency (12.6% and 13.5%). It should be noted that Harada et al. (6) defined pendelluft volume as the total, rather than excess, volume passing through the daughter airways when the flows between two connected segments of the lung are in opposing direction. This is potentially confounding, insofar as anything beyond twice the minimum of the absolute values of flow in the daughter branches is passed to or from the parent airway (14).
In a mechanical model of the respiratory system, High et al. (3) found that the largest pendelluft was 275% of the tidal volume; when the model was driven with a high frequency, resistances were low, inertances were high, and the compliances were very different. The analytical results for RLC circuit limits presented here match the “cross-over frequency” at which the highest pendelluft was observed.
Clear asynchronies in parallel filling were apparent when an excised dog lung was driven at high frequencies by Lehr et al. (the “Disco Lung”) (10). One interpretation of these results rests on the idea of wave propagation, where the gas inertance is coupled to local compliance, resulting in a type of pendelluft closer to underdamped high-frequency RLC circuits. These phenomena nevertheless conform to our general description within the context of inertance-dominated branches, with the resulting spatial patterns reflecting the structure of the tree at the segmental or subsegmental bronchial level. Amini and Kaczka (1) included impedances, gas compression, and airway wall distention in a computational model of the bronchial tree and found frequency-dependent asynchrony among acinar flows, indicating that pendelluft was present.
The pendelluft explored in this study does not consider pendelluft that may emerge as the consequence of an unevenly distributed plural pressure. There is recent evidence supporting that this latter form of pendelluft may emerge during mechanical ventilation in the presence of spontaneous effort (22). Similarly, the beating heart can create local pressure differences that result in pendelluft (17). In addition, nonlinear phenomenon, such as recruitment and derecruitment of alveoli and airways, can also lead to pressure differences throughout the lung that may lead to pendelluft. For example, the sudden opening of an alveolus will most likely demand air from both parallel pathways (i.e., pendelluft) as well as the serial path from the mouth. Although the balance of these volumes might be determined from the mechanical properties of the airway network, these nonlinear sources of pendelluft are not characterized by the present work.
Limitations.
Limitations of the simulation are related to the model simplifications. The model that we used for our simulation example includes a bronchial tree based on generation 4 to 16 of Weibel's morphometric data for airway length and diameter. It is possible that the four more proximal airway generations could influence the pendelluft at certain frequencies and waveforms, but this remains quantitatively unknown. In addition, we have shown analytically (Fig. 5) that heterogeneous compliances can nearly triple the relative pendelluft. However, the challenge for numerical simulations is that the results depend on the interplay between the pattern of bronchoconstriction in the airway tree and the pattern of heterogeneity in compliance of the terminal units. For example, a tree could theoretically have a heterogeneity in resistances that spatially matches the heterogeneity in compliances, leading to a uniform distribution of time constants and little pendelluft.
The diameters and lengths of the airways were derived from an integrative simulation of bronchoconstriction that has been demonstrated to generate ventilation defects similar to those observed in Positron Emission Tomography imaging (19). It can be assumed that the patterns of clustered constriction of airways that cause the ventilation defects are a reasonable approximation of airway behavior in humans. However, asymmetry in branching of airways within the bronchial tree was not included in that model and may be an additional source of pendelluft volume. Also, the waveform of the input affects the pattern of bronchoconstriction that emerges. To compare different waveforms under identical conditions, the diameters were fixed for that numerical simulation.
Tissue resistance or tissue hysteresivity, such as that described by the constant phase model (5), was not considered in the numerical simulations. The inclusion of tissue behavior using spatially homogeneous or heterogeneous parameters or impedances would likely increase or decrease pendelluft to some degree depending on the resulting differences in impedances at airway bifurcations. Unfortunately, experimental measurements of hysteresivity are affected by heterogeneity (2), so that estimates of its contribution to pendelluft would rely on assumptions. However, it should be noted that any impedance that can be described for a fixed frequency with a real and complex part can be used in Eq. 10 for a single-frequency sinusoid.
The presented analysis assumed that the airway walls are rigid and that they do not expand and store additional gas volume. This neglects the notion introduced by Mead of the shunt-capacitance of the airways (11), where the compliance of the airway can become important at high frequencies when the peripheral resistance is high. During high-frequency ventilation, this effect may improve CO2 elimination in certain circumstances by causing a kind of serial pendelluft (13). As this effect is not modeled in the simulation, it is unclear how neglecting the airway compliance might influence pendelluft, particularly at higher frequencies. Also, it should be noted that the frequency used in high-frequency ventilation [up to 900 beats/min or 15 Hz (4)] did not create significant inertial pendelluft in the simulation (Fig. 8).
Summary.
In summary, we generalized the concept of relative pendelluft volume introduced by Otis et al. (12) to include general flow patterns and the entire bronchial tree. We quantified the limits of pendelluft volume at a single bifurcation. Using a numerical example of bronchoconstriction, we illustrated that small-magnitude pendelluft can emerge in asthma and that pendelluft depends on the breathing profile. Although the overall magnitude of pendelluft was small in the example we explored, it was concentrated in poorly ventilated regions of the lung. This preferential site of pendelluft may contribute to local gas exchange, irreversible mixing (3), and aerosol deposition patterns in poorly ventilated regions of the lung.
GRANTS
This work was supported by National Heart, Lung, and Blood Institute of the National Institutes of Health grant R01HL087281.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
Author contributions: E.E.G. and T.W. conception and design of research; E.E.G. and T.W. performed experiments; E.E.G., J.P.B., and T.W. analyzed data; E.E.G., J.P.B., and T.W. interpreted results of experiments; E.E.G. and T.W. prepared figures; E.E.G. and T.W. drafted manuscript; E.E.G., J.P.B., J.G.V., and T.W. edited and revised manuscript; E.E.G., J.P.B., J.G.V., and T.W. approved final version of manuscript.
Glossary
- V̇j, Vj
Flow and tidal volumes of the jth daughter airway in a bifurcation (j = 1,2 for all subscripts j).
- Vk
Tidal volume passing through the kth airway.
- V̇in, Vin
Inlet flow and (tidal) volume to a bifurcation; depending on the context it may be related to the whole airway tree or a subtree.
- V̇P,j
Pendelluft flow in the jth daughter airway of a bifurcation.
- V̇PB,k, VPB,k
Pendelluft flows and volumes at the kth bifurcation. In the context of a local bifurcation, the subscript k is omitted.
- VP
Pendelluft volume in an airway tree (global) or subtree (regional).
- VAP,j
The aggregate pendelluft volume passing through the jth daughter airway in a bifurcation.
- VAP,in
The aggregate pendelluft volume passing through the parent airway of a bifurcation.
- VB,j
The bulk volume that directly passes through the jth airway of a bifurcation without experiencing pendelluft.
- β
The set of bifurcations in an airway tree or subtree.
- T
The set of terminal airways in an airway tree or subtree.
- Rj, Cj, Lj, Zj
The (frequency-dependent) resistance, compliance, inertance, and impedance of the jth airway of a bifurcation and its subtended region.
- τj
Model-dependent nondimensional time constant of the jth airway of a bifurcation and its subtended region.
- κ
Nondimensional compliance or inertance ratio of the daughter airways and their subtended regions.
REFERENCES
- 1.Amini R, Kaczka DW. Impact of ventilation frequency and parenchymal stiffness on flow and pressure distribution in a canine lung model. Ann Biomed Eng 41: 2699–2711, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bates JH. Lung Mechanics: An Inverse Modeling Approach. Cambridge, MA: Cambridge University Press, 2009 [Google Scholar]
- 3.Butler JP, Tsuda A. Logistic trajectory maps and aerosol mixing due to asynchronous flow at airway bifurcations. Respir Physiol Neurobiol 148: 195–206, 2005 [DOI] [PubMed] [Google Scholar]
- 4.Butler W, Bohn D, Bryan A, Froese A. Ventilation by high-frequency oscillation in humans. Anesth Analg 59: 577–584, 1980 [PubMed] [Google Scholar]
- 5.Hantos Z, Daroczy B, Suki B, Nagy S, Fredberg J. Input impedance and peripheral inhomogeneity of dog lungs. J Appl Physiol 72: 168–178, 1992 [DOI] [PubMed] [Google Scholar]
- 6.Harada K, Saoyama N, Izumi K, Hamaguchi N, Sasaki M, Inoue K. Experimental pendulum air in the flail chest. Jpn J Surg 13: 219–226, 1983 [DOI] [PubMed] [Google Scholar]
- 7.High K, Ultman J, Karl S. Mechanically induced pendelluft flow in a model airway bifurcation during high frequency oscillation. J Biomech Eng 113: 342, 1991 [DOI] [PubMed] [Google Scholar]
- 8.Kamm R, Stutsky A, Drazen J. High-frequency ventilation. Crit Rev Biomed Eng 9: 347, 1984 [PubMed] [Google Scholar]
- 9.Lee WJ, Kawahashi M, Hirahara H. Experimental analysis of pendelluft flow generated by HFOV in a human airway model. Physiol Meas 27: 661, 2006 [DOI] [PubMed] [Google Scholar]
- 10.Lehr JL, Butler JP, Westerman PA, Zatz SL, Drazen JM. Photographic measurement of pleural surface motion during lung oscillation. J Appl Physiol 59: 623–633, 1985 [DOI] [PubMed] [Google Scholar]
- 11.Mead J. Contribution of compliance of airways to frequency-dependent behavior of lungs. J Appl Physiol 26: 670–673, 1969 [DOI] [PubMed] [Google Scholar]
- 12.Otis A, Mckerrow C, Bartlett R, Mead J, McIlroy M, Selver-Stone N, Radford E, Jr. Mechanical factors in distribution of pulmonary ventilation. J Appl Physiol 8: 427, 1956 [DOI] [PubMed] [Google Scholar]
- 13.Rossing T, Slutsky A, Ingram R, Kamm R, Shapiro A, Drazen J. CO2 elimination by high-frequency oscillations in dogs–effects of histamine infusion. J Appl Physiol 53: 1256–1262, 1982 [DOI] [PubMed] [Google Scholar]
- 14.Safonoff I, Emmanuel GE. The effect of pendelluft and dead space on nitrogen clearance: mathematical and experimental models and their application to the study of the distribution of ventilation. J Clin Invest 46: 1683, 1967 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sala H, Fernandez A, Guardia S, González A, Rodenstein D. Supramaximal flow in asthmatic patients. Eur Respir J 19: 1003–1007, 2002 [DOI] [PubMed] [Google Scholar]
- 16.Shinozuka N, Sato J, Kohchi A, Nishino T, Mizuguchi T. Pendelluft is not the major contributor to respiratory insufficiency in dogs with flail chest: a mathematical analysis. J Anesth 9: 252–259, 1995 [DOI] [PubMed] [Google Scholar]
- 17.Thurgood J, Dubsky S, Henon Y, Jesudason E, Fouras A. Heart-lung interactions: A vital source of gas mixing within the lungs. Am J Respir Crit Care Med 189: A6275, 2014 [Google Scholar]
- 18.Ultman JS, Shaw RG, Fabiano DC, Cooke KA. Pendelluft and mixing in a single bifurcation lung model during high-frequency oscillation. J Appl Physiol 65: 146–155, 1988 [DOI] [PubMed] [Google Scholar]
- 19.Venegas JG, Winkler T, Musch G, Melo MFV, Layfield D, Tgavalekos N, Fischman AJ, Callahan RJ, Bellani G, Harris RS. Self-organized patchiness in asthma as a prelude to catastrophic shifts. Nature 434: 777–782, 2005 [DOI] [PubMed] [Google Scholar]
- 20.Vyshedskiy A, Murphy R. Pendelluft in chronic obstructive lung disease measured with lung sounds. Pulm Med 2012: 139395, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Winkler T, Venegas JG. Complex airway behavior and paradoxical responses to bronchoprovocation. J Appl Physiol 103: 655–663, 2007 [DOI] [PubMed] [Google Scholar]
- 22.Yoshida T, Torsani V, Gomes S, De Santis RR, Beraldo MA, Costa EL, Tucci MR, Zin WA, Kavanagh BP, Amato MB. Spontaneous effort causes occult pendelluft during mechanical ventilation. Am J Respir Crit Care Med 188: 1420–1427, 2013 [DOI] [PubMed] [Google Scholar]