Abstract
Background
Immediate expander-based breast reconstruction after mastectomy is a prevalent option for many women with breast cancer. When coupled with adjuvant radiation, however, radiation-induced skin and soft tissue injury diminish the success of this reconstructive technique. We hypothesize that prophylactic administration of the cytoprotectant Amifostine will reduce soft tissue complications from irradiation, aiding expander-based reconstruction for women battling this disease.
Methods
Sprague Dawley rats were divided into two experimental groups, Operative Expander Placement (Expander) and Operative Sham (Sham). Expander specimens received a sub-latissimus tissue expander with a 15cc fill volume; Shams underwent identical procedures without expander placement. Experimental groups were further divided into Control specimens receiving no further intervention, XRT specimens receiving human-equivalent radiation, and AMF-XRT specimens receiving both Amifostine and human-equivalent radiation. Animals underwent a 45-day recovery period and were evaluated grossly and via ImageJ analysis for skin and soft tissue complications.
Results
None of the Control, XRT, or AMF-XRT Sham specimens showed skin and soft tissue complications. For Expander animals, significantly fewer AMF-XRT specimens (4 of 13, 30%) demonstrated skin and soft tissue complications compared to XRT specimens (9 of 13, 69%; p = 0.041). ImageJ evaluation of Expander specimens demonstrated a significant increase in skin and soft tissue necrosis for XRT specimens (12.94%), compared with AMF-XRT animals (6.96%, p = 0.019).
Conclusions
Amifostine pre-treatment significantly reduced skin and soft-tissue complications in both gross inspection and ImageJ analysis. These findings demonstrate that Amifostine prophylaxis provides protection against radiation-induced skin and soft tissue injury in a murine model of expander-based breast reconstruction.
Level of Evidence
Animal study, not gradable for level of evidence.
Introduction
Breast cancer will affect 1 out of every 8 women during their lifetime.1 With over 232,000 new diagnoses and 40,000 deaths anticipated in 2013, it is the most common non-cutaneous malignancy and second-most common cause of cancer death for women in the United States.2 However, an increase in social awareness and a clinical consensus on screening standards has led to diagnoses being made at earlier stages of the disease; with earlier detection comes the benefits of significantly improved survival rates and less invasive surgical treatment options.3
In addition to tumor extirpation, adjuvant radiation is a crucial component of many breast cancer treatment regimens. Radiation therapy decreases loco-regional recurrence and increases both disease-free and overall survival.3 However, despite its therapeutic and prognostic benefits, radiation administration is fraught with deleterious consequences such as compromised wound healing, impaired collagen synthesis, and a prolonged inflammatory response; these sequelae can impede and substantially hinder approaches to reconstructive procedures.4,5 Complications with tissue expander-based breast reconstruction performed in concert with radiation are reported as high as 60% and include surgical site infection, expander extrusion, tissue contracture, and unappealing aesthetic outcome, all of which can result in tremendous physical and psychological detriment.4-6
Amifostine is a cytoprotectant currently on formulary for prophylaxis against radiation-induced xerostomia and mucositis in squamous cell head and neck cancer. The active metabolite of Amifostine is a free-radical scavenger that regulates cell cycle checkpoints and gene expression while also facilitating repair of damaged DNA.7,8 The efficacy of radiation therapy is uncompromised by Amifostine administration, and data demonstrate no increased tumor recurrence or decreased disease-free and overall survival when Amifostine is combined with radiation therapy.7-9
We hypothesize that the protective capacity of Amifostine on soft tissues can be extended to a setting of irradiated expander-based breast reconstruction to reduce radiation-induced complications and afford more reconstructive options for women suffering from this devastating disease. To test this hypothesis, this study utilizes a murine model to mimic tissue expander-based breast reconstruction with subsequent irradiation. Our overarching goal is to translate such findings from the bench to the bedside in order to mitigate the corrosive effects of radiation therapy and introduce an innovative treatment paradigm where oncologic principles are satisfied without compromising the reconstructive preferences of women and their surgeons.
Methods
Adult male Sprague Dawley rats (350g) were obtained through the University of Michigan University Lab Animal Medicine (ULAM) department in compliance with their sub-division of the University Committee on the Use and Care of Animals (UCUCA). Group sizes were determined prior to experimentation with the use of nQuery Advisor 7.0 software. Under the assumption that the data would be evaluated using a general linear model with associated analysis of variance with a desired power of 0.80 with a difference between groups of one standard deviation, we required at least five animals per group. Due to the addition of radiotherapy and the potential for skin and soft tissue injury, we increased group sizes accordingly.
Experimental Grouping
Two randomized experimental groups were created consisting of animals receiving operative tissue expander placement (Expander) and animals undergoing an identical operative procedure without expander placement (Sham). Within these group, experimental arms were included, accordingly; Control animals receiving neither irradiation nor Amifostine pre-treatment, animals receiving radiation therapy, alone (XRT), and animals receiving Amifostine pre-treatment prior to radiation therapy (AMF-XRT) (Figure 1). We included Sham specimens to determine whether the operative procedure itself, irrespective of radiation or Amifostine administration, was responsible for any unique postoperative complications. The study is deliberately unbalanced and does not include a Control arm for the Expander group, as expansion with no further intervention does not address our hypothesis or pertain to the clinical model we are attempting to recreate.
Figure 1.
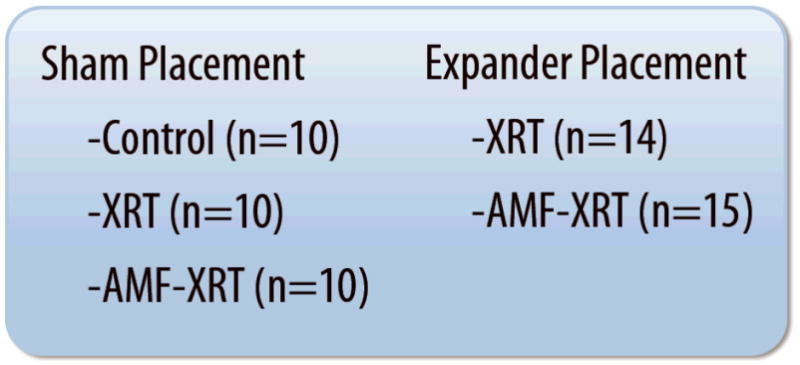
Schematic showing our experimental groups and the arms contained within each. Controls receive no further treatment after their respective procedures. XRT animals receive postoperative radiation, alone, while AMF-XRT animals receive Amifostine pre-treatment prior to radiation administration.
Operative Procedure for Expander & Sham Specimens
Anesthesia induction was accomplished by placing the animal in a sealed chamber with low-flow isoflurane and oxygen. When asleep, the animal was placed on a warming pad and a nose cone attached with silk suture to facilitate isoflurane and oxygen inhalation for the duration of the procedure. Protective ocular lubricant was placed in each eye. Cephazolin (60mg/kg subcutaneous) was administered 45 minutes prior to surgery and every 12 hours, postoperatively, for two additional doses. Buprenorphine (0.04ml subcutaneous) analgesia was administered, along with 25cc/kg subcutaneous Lactated Ringer's solution for perioperative hydration. The right dorsum and posterior midline were shaved and depilatory applied to ensure complete hair removal over an area of approximately 8cm length × 6cm width. A scrub with three alternating solutions of Novalsan and sterile saline was then performed over this area.
The animal was placed prone on a warming pad on the operating table, re-prepped with Novalsan, and draped in sterile fashion. A longitudinal incision was made just right of the dorsal midline, extending 3cm caudally from the inferior angle of the scapula. A 2cm paramedian myotomy was made in the latissimus muscle and a sub-latissimus pocket was created. A sterile, smooth-textured mini-expander (Allergan, Inc., Santa Barbara, CA) measuring 3cm diameter was placed in the sub-latissimus pocket with its port (2cm distal, 1.5cm diameter, 0.6cm height) placed caudally and superficial to the muscle within the subcutaneous space (Figure 2). The latissimus was re-approximated over the expander, skin was closed, and the animal singly-placed in a warmed cage to wake under continuous supervision. For Sham specimens, the above procedure was performed with identical preparation and operative steps save the placement of the mini-expander, itself.
Figure 2.
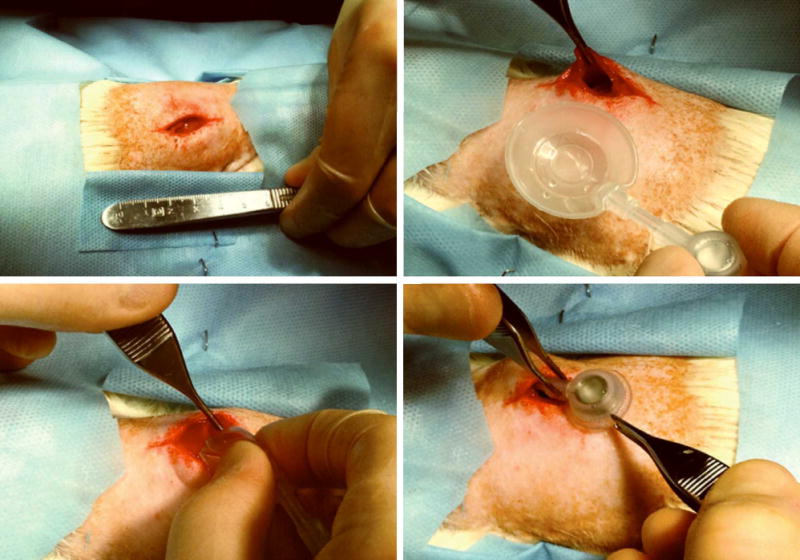
Intra-operative photographs of specimen dorsal midline skin incisions (upper left), expander orientation and placement (upper right and bottom left), and caudal port site placement (bottom right).
Recovery and Expansion
All animals underwent a 14-day postoperative recovery period during which the operative site was monitored for signs of infection, seroma, hematoma, tissue necrosis and breakdown, or expander expulsion. Daily weights were obtained to monitor nutritional status. For Expander specimens, tissue expansion took place under isoflurane drop anesthesia on postoperative days 15, 18, and 21, with 5cc 0.9% normal saline injected during each session to achieve a total fill volume of 15cc (Figure 3). The 15cc total fill volume was determined based on the tension and compliance of the muscle, soft tissue, and skin overlying the implant.
Figure 3.
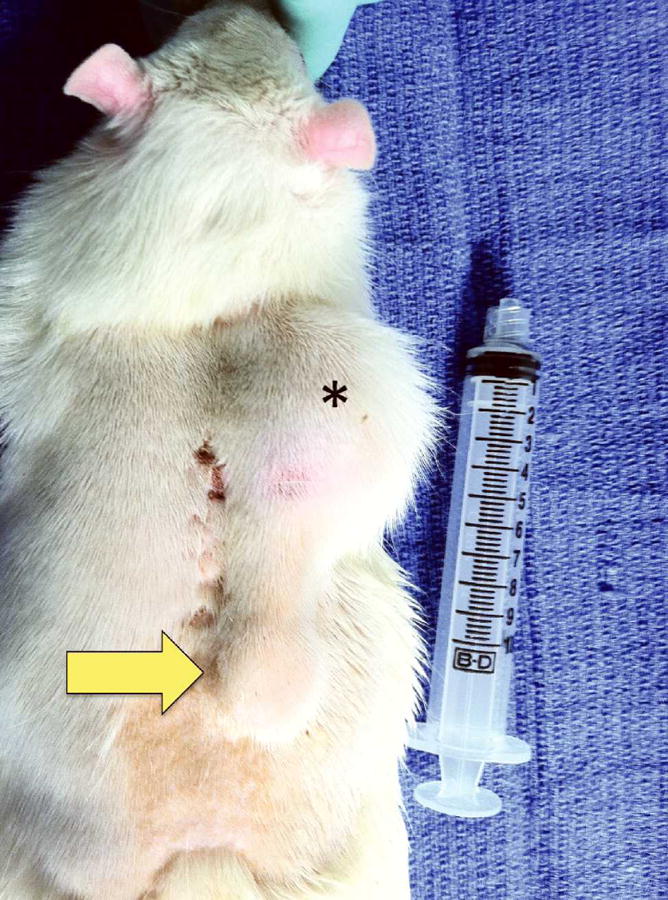
An animal on postoperative day 21 with a 15cc fill volume in the tissue expander. Note the cephalad expander position lateral to the posterior midline (*), and the distal port site (arrow).
Amifostine & Radiation Administration
Postmastectomy radiation therapy typically consists of 50-60Gy administered as 2Gy fractions over 5-6 weeks.10 Collaborating with the Experimental Irradiation Core of the University of Michigan Comprehensive Cancer Center, we calculated a human-equivalent dose radiation regimen of 5 fractions at 5.6Gy/fraction (total 28Gy). Fractions were administered once daily over five consecutive days at a dose rate of 147.7cG/minute using a Philips RT250 orthovoltage unit (250 kV X-rays, 15 mA; Kimtron Medical, Oxford, CT). Rats receiving Amifostine were administered a 100mg/kg dorsal subcutaneous injection 45 minutes prior to radiation, a dose previously derived and utilized by our laboratory in published studies.11,12 Just prior to radiation administration, animals underwent closed chamber induction with inhalational isoflurane. The animal was then placed in the radiation chamber and covered with a protective lead shield that exposed only a 4cm diameter circular region directly overlying the expander. Continuous low-flow isoflurane and oxygen were administered throughout the duration of radiation, which lasted approximately 4 minutes for each fractionated dose. Animals were then singly-placed in cages to wake under continuous supervision.
Observation & ImageJ Analysis
We inspected operative and irradiated sites daily for 45 days from the completion of radiation to evaluate for any erythema, necrosis, alopecia or tissue thinning, expander extrusion, or any other signs of compromised skin and soft tissue integrity. Digital images were collected at weekly intervals to record the quantity and quality of any observed complications. Animals were sacrificed at postoperative day 45, their dorsums shaved, and a 6cm length × 4cm width region of interest subjected to analysis. This region of interest was fixed and consistent for each animal and consisted of the skin and soft tissues surrounding the irradiated operative site and entire tissue expander. ImageJ (U.S. National Institute of Health, Bethesda, MD) technology was used to analyze this region of interest by splining any areas within the region that demonstrated skin or soft tissue damage. The number of pixels in these splined segments was divided over the total number of pixels within the region of interest to result in a quantitative calculation for the percent of skin and soft tissue damage present.13
Statistics
Statistical analysis was performed using a t-test for calculating the percent of skin and soft tissue affected for our ImageJ data. Statistics for all other variables were calculated using a two-sample Mann-Whitney U test. Significance was defined as p < 0.05.
Results
Sham Specimens
During the 45-day recovery period after radiation administration, we saw no evidence of skin or soft tissue destruction, wound breakdown, or any other gross complications in the Sham Control specimens, Sham XRT specimens, or Sham AMF-XRT specimens. The lack of complications within these Sham specimens was a telling finding, indicating that expansion, radiation administration, and prophylactic Amifostine administration were the main determinants of surgical site complication. Given the overall absence of skin and soft tissue injury, ImageJ calculations were not performed for any of the Sham specimen experimental groups.
Expander Specimens
9 of 14 Expander XRT specimens demonstrated substantial skin and soft tissue changes that included erythema, necrosis, hematoma/seroma formation, and alopecia/tissue thinning. In addition, expander port site extrusion was noted for two animals, and a loss of volume after expansion was noted for one animal; volume loss was determined to be from port site and expander separation, and this specimen was not included in our final results. Expander AMF-XRT specimens demonstrated skin and soft tissue complications that included erythema, necrosis, and alopecia/tissue thinning. Additionally, loss of volume after expansion was noted for two animals; similar to the XRT specimens, volume loss was due to port site and expander separation, and these two specimens were not included in final results. Table 1 summarizes these findings, with 9 of 13 (69%) XRT specimens, and 4 of 13 (31%) AMF-XRT specimens displaying some degree of skin and soft tissue injury. The complication rate in AMF/XRT specimens was significantly decreased compared to the XRT specimens (p = 0.041). Figure 4 demonstrates representative specimens displaying the observed complications.
Table 1.
Table demonstrates a reduction of the total complication rate by more than one-half in the Expander AMF-XRT group compared to the Expander XRT group. Specifically, complications of extrusions and hematoma/seroma were eliminated in the AMF-treated group.
| XRT (n=14) | AMF-XRT (n=15) | |
|---|---|---|
| Necrosis | 6 (43%) | 4 (27%) |
| Erythema | 7 (50%) | 4 (27%) |
| Extrusion | 2 (14%) | 0 (0%) |
| Alopecia/Tissue Thinning | 8 (57%) | 1 (7%) |
| Hematoma/Seroma | 2 (14%) | 0 (0%) |
| Total Complications | 9/13 (69%) | 4/13 (31%) |
Figure 4.
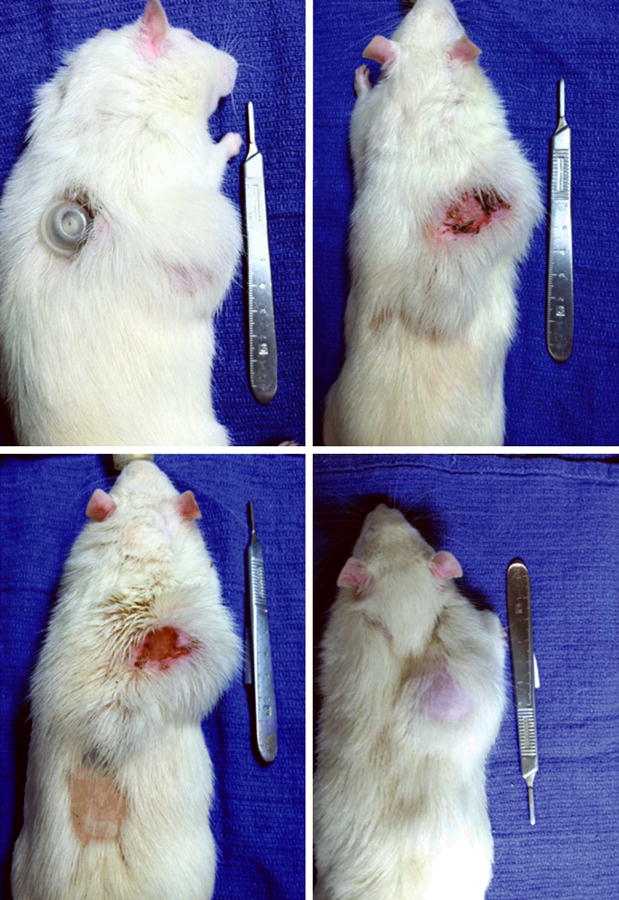
Representative skin and soft tissue complications seen in the Expander specimens. Observed complications included expander port site extrusion (upper left), erythema and necrosis (upper right and bottom left), and alopecia/tissue thinning (bottom left and bottom right). Amifostine pre-treated specimens displayed significantly fewer complications than specimens receiving radiation alone (30% vs. 69%, respectively, p = 0.041).
In addition to evaluating the gross appearance of irradiated operative sites, ImageJ technology was utilized to quantitatively analyze skin and soft tissue injury within a 6cm length×4cm width region of interest surrounding the irradiated field and expander (Figure 5). Graphical representation of the calculated areas of skin and soft tissue damage for each specimen is shown in Figure 6. The mean area affected by radiation-induced damage was significantly less for Amifostine pre-treated animals (6.96%) compared with specimens receiving radiation, alone, (12.94%, p = 0.019), illustrated in Figure 7.
Figure 5.
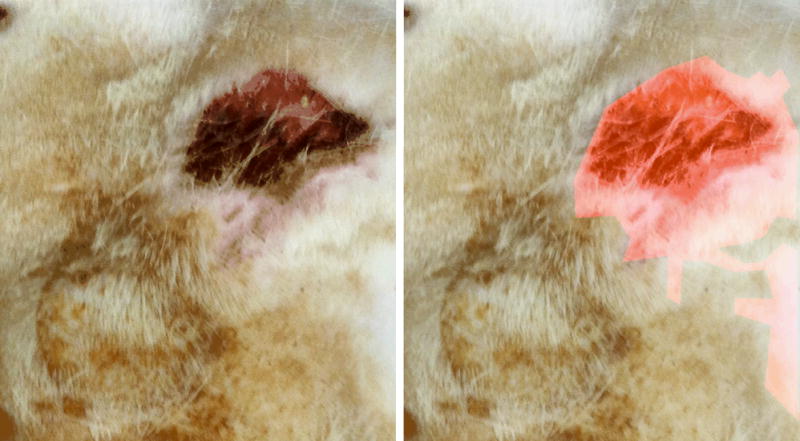
ImageJ evaluation of the 6cm length × 4cm width region of interest. This specimen shows erythema, necrosis, and alopecia/tissue thinning within the region of interest. The left image is the original gross picture, while the right image is modified with color to delineate areas of skin and soft tissue injury.
Figure 6.
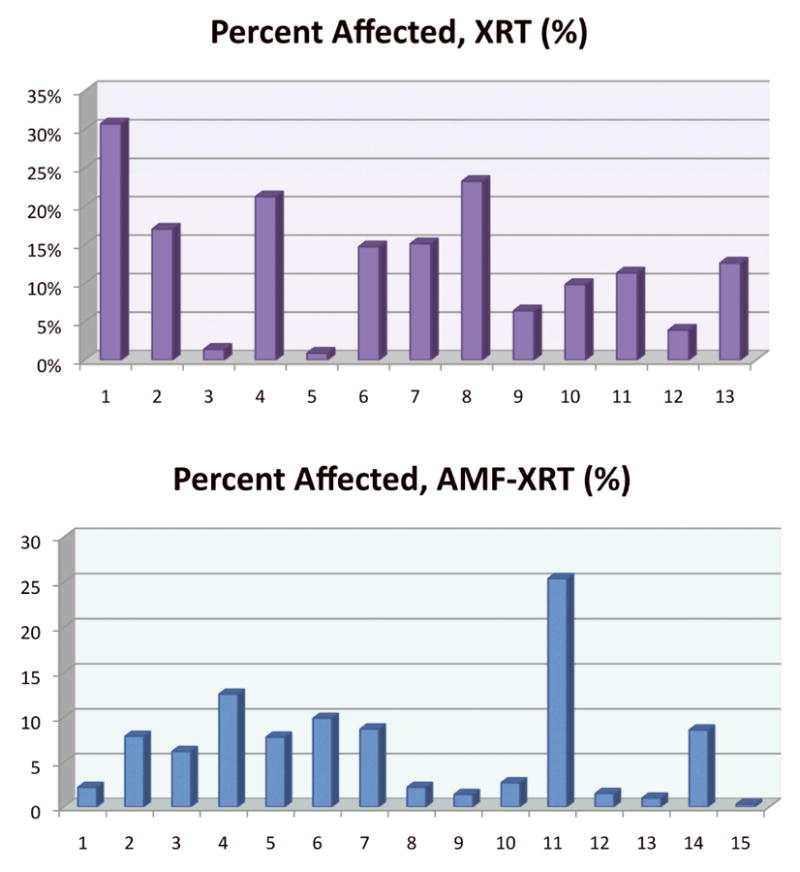
Graphical representation showing the percent of skin and soft tissue affected by radiation-induced injury for Expander XRT and Expander AMF-XRT specimens. The percent calculation was derived from ImageJ analysis of a 6cm length × 4cm width region of interest containing the irradiated operative site.
Figure 7.
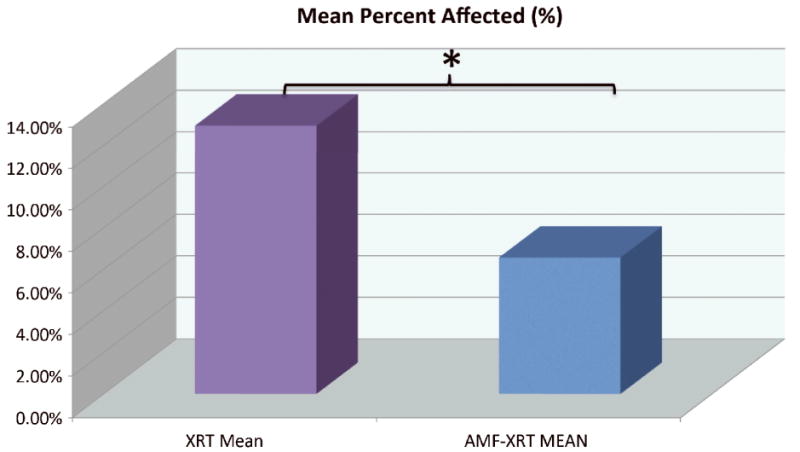
Comparing mean percent area of skin and soft tissue necrosis between Expander XRT and Expander AMF-XRT specimens. AMF-XRT specimens demonstrated significantly less necrosis as a group (6.96%) compared with XRT specimens (12.94%, p = 0.019).
Discussion
Adjuvant radiation therapy is a necessary component of treatment regimens for many patients with breast cancer. While performed with the goal of decreasing tumor recurrence and optimizing survival, the deleterious effects of radiation result in staggeringly high rates of morbidity and pose considerable challenges to the surgeon undertaking the task of reconstruction.4-6,14
Satisfactory breast reconstruction after mastectomy is a crucial factor in the physical and emotional recovery of patients.6,15,16 However, reconstructive efforts performed in either immediate or delayed fashion are compromised when undertaken juxtaposed to radiation administration. Benefits of immediate reconstruction, typically involving implant placement performed in the same operative setting as mastectomy, include shorter total operative times, improved cosmesis, and the advantage of working with tissues unaffected by prior irradiation.17 Still, when coupled with postoperative radiation administration, these reconstructions carry an increased incidence of wound breakdown, skin necrosis, implant exposure, and overall operative failure rates of 21-60%.6 Furthermore, nearly one third of patients will require operative revision due to radiation-induced complications.6,18 Delayed reconstruction methods, performed utilizing autologous flaps or exchanging tissue expanders for permanent implants, carry a 50% complication rate due to radiation-induced wound breakdown, contracture, or overall flap failure.17,18 Regardless of the timing or selected operative approach, the necessity of radiation therapy leads to increased complications, lessens the potential for an acceptable cosmetic result, and diminishes overall patient and physician satisfaction with respect to the final reconstruction.4,6,15-17,19,20
Based on prior laboratory studies demonstrating radioprotective properties and the current indications for prophylaxis against radiation-induced soft tissue injury, we hypothesized that Amifostine administration could be extended to tissue expander-based breast reconstruction coupled with adjuvant radiation. In this setting, the potential exists to ameliorate associated complications of radiation administration while affording more reconstructive options for women suffering from this devastating disease. To test our hypothesis, we utilized a murine model to mimic tissue expander-based breast reconstruction with irradiation. None of the sham-exposed animals demonstrated any appreciable skin or soft tissue injury, indicating that expansion, radiation administration, and prophylactic Amifostine administration were the potential determinants of complication and remediation. With respect to Expander specimens, a marked difference existed between XRT and AMF-XRT animals when evaluating for the incidence of alopecia/tissue thinning, erythema, necrosis, hematoma/seroma formation, and expander extrusion; a significant decrease in gross skin and soft tissue complications was seen in the AMF-XRT specimens compared with the XRT specimens (31% and 69%, respectively, p = 0.014). When evaluating quantitative metrics of the irradiated operative sites with ImageJ, the mean value of persistent necrosis across XRT specimens was nearly double that of the AMF-XRT specimens (12.94% and 6.96%, respectively, p = 0.019). The diminished incidence of these complications in Expander AMF-XRT specimens suggests a strong correlation between Amifostine administration and a diminution of the negative consequences of irradiation.
The reduced rate of post-irradiation skin and soft tissue complications and necrosis in Amifostine pre-treated specimens is both statistically and clinically significant; successful radioprotection would improve clinical practice and potentially allow for a full array of reconstructive options for the treatment of women with breast cancer. It would also provide an operative field free from the technical challenges associated with radiation-induced injury, potentially resulting in diminished rates of reconstructive failure or necessity for re-operation, and ultimately resulting in improved patient and physician satisfaction.
The findings of this study have the potential to drastically impact upon the operative management of patients with breast cancer. Regardless as to the type or timing of reconstruction, any operative intervention coupled with a treatment regimen utilizing radiation therapy is at risk for detrimental radiation-induced sequelae. While this experiment specifically focuses on a model of irradiated expander-based breast reconstruction, it is not unreasonable to suggest that all patients receiving radiation therapy might benefit from prophylactic Amifostine in order to protect and preserve normal, native skin and soft tissues from the untoward effects of radiation. With respect to translating our current experimental findings to future clinical application, we anticipate that the dose and route of Amifostine administration would be consistent with current formulary guidelines and previously published regimens.8 The drug is available both as an injectable solution and as a powder for injection. 200mg/m3 is injected intravenously via peripheral or central venous access catheters over a 3-minute infusion period. This would be performed 15 to 30 minutes before each round of administered XRT.
It is important to address the limitations of this current study. As it currently stands with our experimental groups being deliberately unbalanced, this study does not parse the degree of complication due to expander placement alone vs. expander placement within an irradiated field. While that comparison itself may make for an interesting investigation, such an inquiry was neither our purpose nor a key portion of our hypothesis. We made a concerted decision to omit such a group in this model and focused rather on determining what protection, if any, Amifostine would impart to the skin and soft tissues for women with breast cancer undergoing immediate tissue expander-based reconstruction and radiation administration. Our study's aim was not to determine whether expander placement and expansion itself would cause wound detriment, but rather, in a setting of expander placement and expansion in an irradiated field, can Amifostine ameliorate anticipated and deleterious sequelae. We are confidant that our results and conclusions demonstrate that it indeed can. In fact, it could be suggested that our findings are all the more meaningful given that we were able to prevent the development of complications potentially attributable to both the placement of an expander subsequent expansion and the administration of radiation. Nevertheless, we are now establishing future studies that will include an arm of non-radiated and non-treated expanded controls to determine the extent of complications due to irradiation compared with non-radiated expander placement and expansion. Furthermore, a 15cc expander fill volume was used uniformly across all animal specimens based on overlying skin tension. Varying the fill volume may further influence the rates and types of complications seen across experimental groups and add statistical significance to these findings.
Another study limitation is that gross inspection of the operative site was a main determinant of skin and soft tissue breakdown, and although the analyses used were controlled and objectively ascertained, researchers were not blinded to the experimental groups. While it would have been possible to randomize our photo documentation and ImageJ figures in order for blinded investigators to evaluate and calculate additional areas of skin and soft tissue pathology, there is a significant level of expertise required for such an endeavor given the technical and time-sensitive aspects of performing such a task. We are currently in the process of training independent observers with the goal of employing randomization and blinding for animal specimens in future studies, which we anticipate will provide substantiation with respect to our processes of both gross and ImageJ analysis.
Future additional analysis with vessel perfusion studies, histomorphometry, and establishing dose-response studies for both irradiation and Amifostine may also build upon the meaningful findings of this study. We are currently in the process of adding even more specimen numbers and working on computer-derived vascular measures to give a true quantitative measurement of vascularity that is superior to the semi-qualitative measures used thus far. Other future study endpoints include investigations to analyze the histological parameters of fibrosis and tissue thinning in the area overlying the irradiated expander, as well as the incidence of radiation-induced capsular contracture. Ideally, quantitative analysis should be sought for defining and determining these parameters, something heretofore that has been difficult in assessing capsular formation; however, similar to the utilization of gross and ImageJ evaluation in our current study, qualitative analysis may also be included, the findings for which and would certainly be of interest.
The fields of general surgery, plastic surgery, and radiation oncology may all potentially benefit from the implementation of Amifostine in treatment regimens utilizing radiation administration for women with breast cancer. The biomedical burden imposed by this terrible disease, the ravaging effects of radiation on normal skin and soft tissues, and the imperfect reconstructive options for patients suffering with this malady mandate a focused line of inquiry into solving this complex and clinically relevant problem.
Conclusion
The corrosive affects of radiation therapy are a critical barrier to progress for physicians contending with the scourge of adverse outcomes in tissue expander-based breast reconstruction. We have shown that Amifostine prophylaxis significantly diminishes the rate of radiation-induced skin and soft tissue complication in this murine model. The implication of these findings is that Amifostine provides substantial protection against radiation-induced skin and soft tissue injury, and has the potential to play an important role in reconstructive efforts for women with breast cancer undergoing treatment regimens utilizing radiation therapy.
Acknowledgments
We wish to thank Dr. Ted Lawrence, Dr. Mary Davis, Dr. David Karnak, and the rest of our collaborators in the Experimental Irradiation Core of the University of Michigan Comprehensive Cancer Center for help with calculating and administering radiation doses to the animal specimens. We would also like to thank Dr. Edwin G. Wilkins and Dr. David L. Brown for their assistance with our expansion protocol. Lastly, we are grateful to Allergan, Inc., for their assistance with supplying the expanders used in this study.
Funding supported by the following grant: “Optimization of Bone Regeneration in the Irradiated Mandible”, NIH-R01#CA 125187- 01, PI: Steven R. Buchman.
Footnotes
Allergan, Inc. supplied the expanders used in this study and had the opportunity to review the final version of this manuscript to address any factual inaccuracies.
Financial Disclosure and Products: None of the authors has a financial interest in any of the products, devices, or drugs mentioned in this manuscript.
References
- 1.Howlader N, Noone AM, Krapcho M, et al. SEER Cancer Statistics Review 1975-2009, (Vintage 2009 Populations) National Cancer Institute; Bethesda, MD: 2012. [Google Scholar]
- 2.American Cancer Society. Data provided as breast cancer facts& figures, 2013. [Accessibility verified October 20, 2013]; Available at www.cancer.org.
- 3.Cameron JL. Current Surgical Therapy. 9th. Elsevier Mosby; 2008. p. 647. [Google Scholar]
- 4.Kronowitz SJ, Robb GL. Breast reconstruction with postmastectomy radiation therapy: Current issues. Plastic and Reconstructive Surgery. 2004;114:950. doi: 10.1097/01.prs.0000133200.99826.7f. [DOI] [PubMed] [Google Scholar]
- 5.Krueger EA, Wilkins EG, Strawderman M, Cederna P. Complications and patient satisfaction following expander/implant breast reconstruction with and without radiotherapy. Int J Radioation Oncology Biol Phys. 2001;41(3):713. doi: 10.1016/s0360-3016(00)01402-4. [DOI] [PubMed] [Google Scholar]
- 6.Kronowitz SJ, Lam C, et al. A multidisciplinary protocol for planned skin-preserving delayed breast reconstruction for patients with locally advanced breast cancer requiring postmastectomy radiation therapy: 3-year follow-up. Plastic and Reconstructive Surgery. 2011;127(6):2154. doi: 10.1097/PRS.0b013e3182131b8e. [DOI] [PubMed] [Google Scholar]
- 7.Andreassen CJ, Grau C, Lindegaard JC. Chemical radioprotection: A critical review of amifostine as a cytoprotector in radiotherapy. Seminars in Radiation Oncology. 2003;13(1):62. doi: 10.1053/srao.2003.50006. [DOI] [PubMed] [Google Scholar]
- 8.Brizel DM, Wasserman TH, et al. Phase III randomized trial of Amifostine as a radioprotector in head and neck cancer. Journal of Clinical Oncology. 2000;18(19):3339. doi: 10.1200/JCO.2000.18.19.3339. [DOI] [PubMed] [Google Scholar]
- 9.Wasserman TH, Brizel DM, et al. Influence of intravenous Amifostine on xerostomia, tumor control, and survival after radiotherapy for head-and-neck cancer: 2-year follow-up of a prospective, randomized, phase III trial. International Journal of Radiation Oncology*Biology*Physics. 2005;63(4):985. doi: 10.1016/j.ijrobp.2005.07.966. [DOI] [PubMed] [Google Scholar]
- 10.National Cancer Comprehension Network. [Accessibility verified October 20, 2013]; Available at www.NCCN.org.
- 11.Tchanque-Fossuo CN, Donneys A, et al. Amifostine remediates the degenerative effects of radiation on the mineralization capacity of the murine mandible. Plastic and Reconstructive Surgery. 2012;129(4):646. doi: 10.1097/PRS.0b013e3182454352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Monson LA, Farberg AS, Jing XL, Tchanque-Fossuo CN, Donneys A, Buchman SR. Distraction osteogenesis in the rat mandible following radiation and treatment with amifostine. Plastic and Reconstructive Surgery. 2010;125(6):41. [Google Scholar]
- 13.Rasband WS. ImageJ, US. National Institutes of Health; Bethesda, Maryland, USA: 1997-2012. http://imagej.nih.gov/ij/ [Google Scholar]
- 14.Bondurant S, Ernster V, Herdman R. Institute of Medicine (US) Committee on the Safety of Silicone Breast Implants. Washington (DC): National Academies Press (US); 1999. Safety of Silicone Breast Implants; p. 5. Reoperations and Specific Local and Perioperative Complications. [PubMed] [Google Scholar]
- 15.Cordeiro PG, Pusic AL, et al. Irradiation after immediate tissue expander/implant breast reconstruction: Outcomes, complications, aesthetic results, and satisfaction among 156 patients. Plastic and Reconstructive Surgery. 2004;113(3):877. doi: 10.1097/01.prs.0000105689.84930.e5. [DOI] [PubMed] [Google Scholar]
- 16.Harcourt DM, Rumsey NJ, et al. The psychological effect of mastectomy with or without breast reconstruction: A prospective, multicenter study. Plastic and Reconstructive Surgery. 2003;111(3):1060. doi: 10.1097/01.PRS.0000046249.33122.76. [DOI] [PubMed] [Google Scholar]
- 17.Rogers NE, Allen RJ. Radiation effects on breast reconstruction with the deep inferior epigastric perforator flap. Plastic and Reconstructive Surgery. 2002;109(6):1919. doi: 10.1097/00006534-200205000-00022. [DOI] [PubMed] [Google Scholar]
- 18.Tran NV, Chang DW, et al. Comparison of immediate and delayed free TRAM flap breast reconstruction in patients receiving postmastectomy radiation therapy. Plastic and Reconstructive Surgery. 2000;108(1):78. doi: 10.1097/00006534-200107000-00013. [DOI] [PubMed] [Google Scholar]
- 19.Goodman CM, et al. Radiotherapy: Effects on expanded skin. Plastic and Reconstructive Surgery. 2002;110(4):1080. doi: 10.1097/01.PRS.0000020992.77582.C2. [DOI] [PubMed] [Google Scholar]
- 20.Clugston PA, et al. A rat model for capsular contracture: The effects of surface texturing. Annals of Plastic Surgery. 1994;33(6):595. doi: 10.1097/00000637-199412000-00005. [DOI] [PubMed] [Google Scholar]


