Figure 6.
Structural Rearrangement of eIF2α in Formation of the py48S
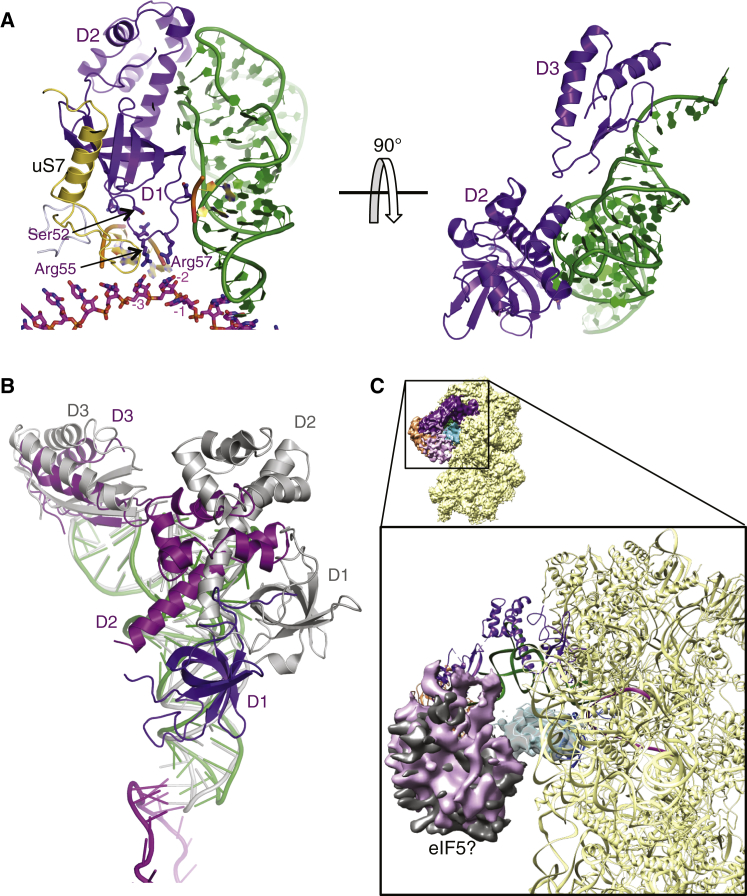
(A) Interaction of the three domains (D1, D2, and D3) of eIF2α with tRNAi and mRNA. Arg55 and Arg57 are shown interacting with mRNA at positions −3 and −2. The conserved Ser52 that is the target of phosphorylation is also shown as sticks.
(B) Structural rearrangement of D1 and D2 of eIF2α (shown in different shades of violet) in the py48S compared to their conformations in the isolated aIF2 ternary complex (gray; Schmitt et al., 2012).
(C) Density potentially from eIF5 (pink), connecting eIF1 (cyan), and eIF2γ (orange). Similar density (gray) is found in the PIC-2 complex. Both densities are low-pass filtered to 8 Å.
See also Figure S7.