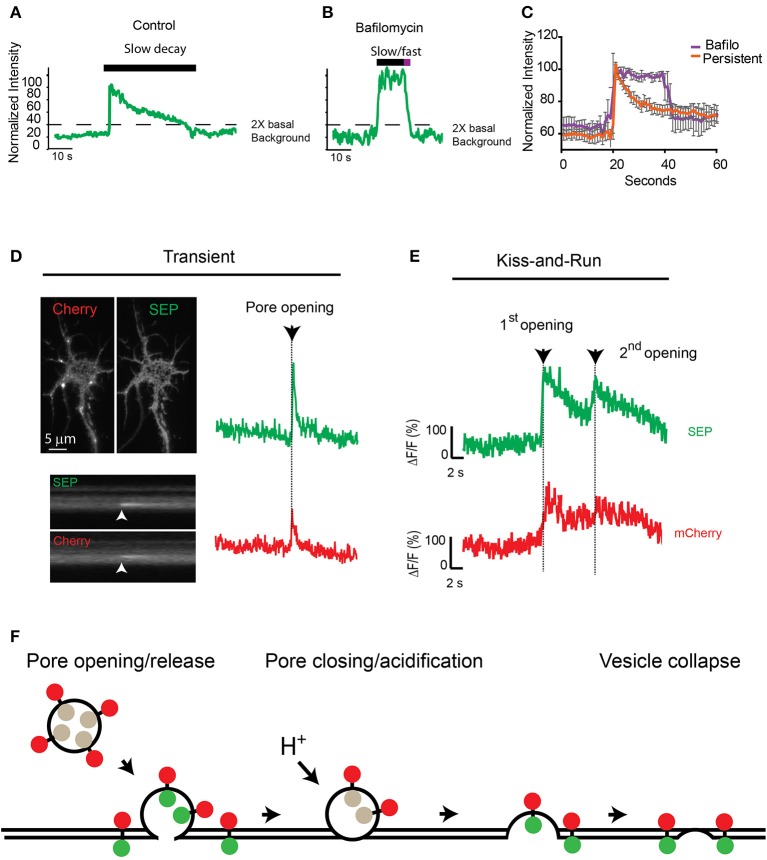
Figure 4.
Consecutive transferrin exocytosis is mediated by a kiss-and-run mechanism. (A) Intensity measurements from control persistent events show slow decay kinetics (>3 s from maximum intensity to background level). (B) Neurons preincubated with 0.5 μM bafilomycin showed persistent events with an initial plateau followed by a fast faster decay kinetics (<3 s from maximum intensity to background levels). (C) Analysis of multiple persistent events from control and bafilomycin treated neurons indicate that bafilomycin created biphasic events with an initial plateau followed by a fast exponential decay (half-life ~2 s). Persistent events from control cells presented a single slow exponential decay (half-life ~17 s, see Figure 2A), errors represent standard error of the mean. (D) mCherry-TfR-SEP was transfected into hippocampal cultures and imaged with simultaneous dual-color TIRF. Kymographs show a transient exocytotic event in the green (SEP) and red (mCherry) channels. Maximum intensity measurements show fusion pore opening and cargo release. Arrow indicates exocytosis. (E) Fluorescence intensity from mCherry-TfR-SEP persistent event. Red trace (mCherry) indicates receptors are retained inside the exocytotic vesicle after an initial release to the cell surface (1st opening). Only after the second fusion pore opening, which was observed in both channels, mCherry levels reached background levels, indicating receptor release. (F) Exocytotic vesicles transporting mCherry-TfR-SEP are visible in the red channel (mCherry), but they are invisible in the green channel (SEP) before fusion. SEP intensity increases rapidly when the vesicular content is neutralized and mCherry-TfR-SEP is partially released onto the plasma membrane. Receptors remaining in the exocytotic vesicle are visible in the red channel. Bafilomycin prevents acidification and the decrease in SEP fluorescence.