Fig. 1.
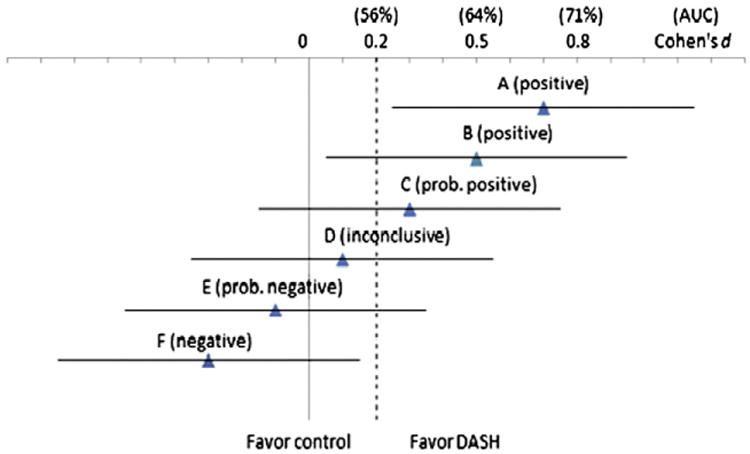
Illustrative scenarios (A–F) of pilot study results for the primary outcome — ACQ score: effect size estimates are expressed in Cohen's d together with the expected 2-sided 95% confidence intervals (standardized half-width = 0.45 at n = 45/arm with a projected 10% attrition over 6 months). AUC (area under the receiver operating characteristic curve) values are given to indicate standards for assessing clinical significance corresponding to those for d. AUC measures the probability that a randomly selected subject in the intervention has a better response than a randomly selected subject in the control [122–124]. Above each line in parentheses is the decision concerning whether a full trial is warranted.
