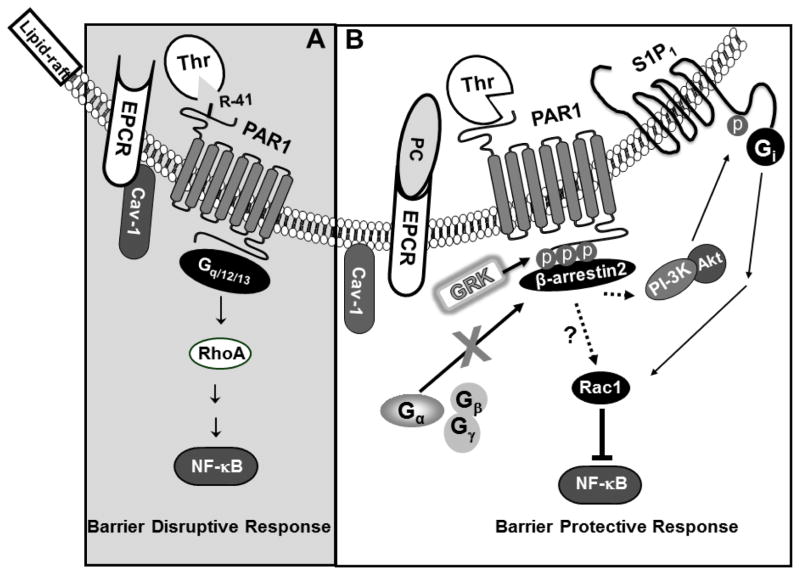
Fig. 2.
Cartoons of PAR1 activation by thrombin in the membrane lipid-rafts of endothelial cells when EPCR is free or occupied by its natural ligand. (A) EPCR interacts with caveolin-1 (Cav-1) within lipid-rafts of endothelial cells when it is not occupied by the Gla-domain of protein C. Under these conditions, thrombin cleavage of PAR1 activates RhoA, up-regulates the NF-κB pathway and elicits disruptive signaling responses through activation of G12/13 and/or Gq proteins. (B) The occupancy of EPCR by protein C leads to dissociation of EPCR from caveolin-1 and a switch in the PAR1-dependent specificity of thrombin signaling, presumably through β-arrestin2 biased signaling and S1P1 transactivation mechanisms as observed with APC signaling.