Abstract
Pediatric complex regional pain syndrome (P-CRPS) offers a unique model of chronic neuropathic pain as it either resolves spontaneously or through therapeutic interventions in most patients. Here we evaluated brain changes in well-characterized children and adolescents with P-CRPS by measuring resting state networks before and following a brief (median = 3 weeks) but intensive physical and psychological treatment program, and compared them to matched healthy controls. Differences in intrinsic brain networks were observed in P-CRPS compared to controls before treatment (disease state) with the most prominent differences in the fronto-parietal, salience, default mode, central executive, and sensorimotor networks. Following treatment, behavioral measures demonstrated a reduction of symptoms and improvement of physical state (pain levels and motor functioning). Correlation of network connectivities with spontaneous pain measures pre- and post-treatment indicated concomitant reductions in connectivity in salience, central executive, default mode and sensorimotor networks (treatment effects). These results suggest a rapid alteration in global brain networks with treatment and provide a venue to assess brain changes in CRPS pre- and post-treatment, and to evaluate therapeutic effects.
Keywords: Children, Resting state, Salience, fMRI, Pain
Highlights
-
•
Pediatric complex regional pain syndrome (CRPS) alters brain networks.
-
•
Alterations are not equivalent to those observed in adults with CRPS.
-
•
Identifying alterations might be relevant to determine treatment modalities.
-
•
Intensive physical- and psycho-therapy reverses some but not all the changes.
1. Introduction
Complex regional pain syndrome (CRPS) is a neuropathic pain condition affecting the peripheral and central nervous system (Marinus et al., 2011) characterized by the continuing presence of pain that is disproportionate to the inciting event. It is frequently accompanied by blood flow and sweating changes, edema, and trophic changes of the skin and subcutaneous tissue in the affected region (Bruehl et al., 1999). Clinical data support the notion of altered changes in CNS processing in CRPS including pain progression (Maleki et al., 2000), movement disorders (Verdugo and Ochoa, 2000) and altered higher-level functions like poor visuo-spatial perception (Sumitani et al., 2007), neglect-like symptoms (inattention, avoid using affected limb) (Galer et al., 1995; Galer and Jensen, 1999; Frettloh et al., 2006; Maihöfner and Birklein, 2007; Punt et al., 2013), altered perception (Peltz et al., 2011), emotional distress (Nagler, 2010) and cognitive dysfunction (Maihöfner and DeCol, 2007). Functional imaging studies in pediatric CRPS patients (Lebel et al., 2008; Linnman et al., 2013) have indicated abnormal brain activity to mechanical (brush) and thermal allodynia (cold) with larger activity than the normal side in sensorimotor, cingulate, and insula cortices, and decreased activity in prefrontal cortex hippocampal and parahippocampal areas. In adults, connectivity analysis resulted in reduction of functional default mode network connectivity in patients vs. controls and increased connectivity of sensorimotor areas with emotional processing brain structures as well as gray matter atrophy in insula, prefrontal cortex, and nucleus accumbens, and changes in white matter fiber integrity in the cingulum-callosal bundle (Maihöfner et al., 2006; Geha et al., 2008; Maihöfner and Peltz, 2011; Bolwerk et al., 2013).
Brain networks define our behaviors in health and disease (Fornito and Bullmore, 2012). Differences in resting state brain networks have been reported across numerous neurological conditions including, depression (Pannekoek et al., 2014), chronic pain (Cauda et al., 2010), anxiety (Bijsterbosch et al., 2014) as well as responses to treatments (McCabe and Mishor, 2011; Posner et al., 2013) including psychologically based treatments (Hashmi et al., 2014). Such measures may also predict the severity of the disease state (Meng et al., 2014). As such the major alterations, common to individuals in the group, are the underlying basis for the altered behavioral phenotype (e.g., pain vs. no pain).
Neuroimaging techniques have allowed the evaluation and determination of such networks. With functional MRI (fMRI), intrinsic brain networks are determined from low-frequency fluctuations of the BOLD signal (Beckmann et al., 2005; De Luca et al., 2006; Lee et al., 2013). It has been used to characterize a number of chronic brain-related diseases including depression (Bohr et al., 2012), schizophrenia (Karbasforoushan and Woodward, 2012), and multiple sclerosis (Filippi et al., 2013) in contrast to healthy brain states. Alterations in intrinsic brain networks have been observed among chronic pain patients with diabetic neuropathy (Cauda et al., 2010), fibromyalgia (Napadow et al., 2010), musculoskeletal pain (Duke Han et al., 2013), and chronic low back pain (Balenzuela et al., 2010; Loggia et al., 2013). The assessment of intrinsic brain network changes in both disease and post-treatment states allows for the determination of alterations and potential normalizations of specific brain area networks. Identification of altered intrinsic brain networks can be used to define treatment targets, and the evolution of these changes can be studied longitudinally with the hypothesis that brain network normalization is a biomarker for disease treatment/control as suggested by Fox and Greicius (2010).
Many pediatric patients with CRPS typically recover with standard medical treatment (Low et al., 2007). In this study, we evaluated intrinsic brain network measures in the P-CRPS disease state among patients who have been resistant to outpatient multidisciplinary care and have been enrolled in an intensive multidisciplinary pain treatment program. This program provides significant clinical benefits following a short (approximately 3-week) rehabilitation program (Logan et al., 2012). In this study, we report measurements of brain network alterations in CRPS pediatric patients before and after treatment as well as compared to a matched healthy control group. We also determined the correlation of brain network changes with psychophysical measures of spontaneous pain pre- and post-treatment. We hypothesized that (1) altered resting state networks in pediatric CRPS patients would resemble the adult condition, (2) with clinical improvement there would be a trend or reversal of alterations in RSNs in the disease condition compared with healthy controls, and (3) that spontaneous pain ratings will correlate with specific brain network connectivities. We suggest that a reversal of brain network alterations to a normative state might be indicative of an individual's brain ability to recover. While this is more likely in children, having imaging measures that define recovery (Maihöfner et al., 2004; Becerra et al., 2009) may be used to identify potential responders and non-responders in the more treatment-resistant adult CRPS population (Azari et al., 2012).
The data suggest that there are significant alterations in networks pre-treatment that for the most part resolve following treatment, with some network changes over-compensating the initial, pre-treatment differences.
2. Materials and methods
2.1. Subjects
The study was approved by the Boston Children's Hospital Institutional Review Board (IRB). The study also met the Helsinki criteria for the study of pain in humans (http://www.wma.net/en/30publications/10policies/b3/). Twenty-six CRPS patients between the ages of 10–18 years with unilateral CRPS of the lower extremity were identified from the Pediatric Pain and Rehabilitation Center (PPRC) at Boston Children's Hospital at Waltham (MA, USA), an intensive interdisciplinary pediatric pain rehabilitation program. Twelve qualified and agreed to participate in this study (Fig. 1). Twelve healthy control participants were recruited through advertisements posted on the web and in local community centers. The healthy controls were age and sex-matched individually to the CRPS patients. All participants were right-handed. Selection criteria: (1) (only for patients) diagnosis of CRPS as determined by an experienced neurologist on the basis of neurological examination and comprehensive record review; (2) no other neurological illness, severe medical problems (such as uncontrolled asthma, acute cardiac disease) or severe psychiatric problems; (3) absence of magnetic implants of any type; (4) no current pregnancy; (5) no history of claustrophobia; (6) weight <285 lbs (130 kg) (the limit of the MRI table).
Fig. 1.
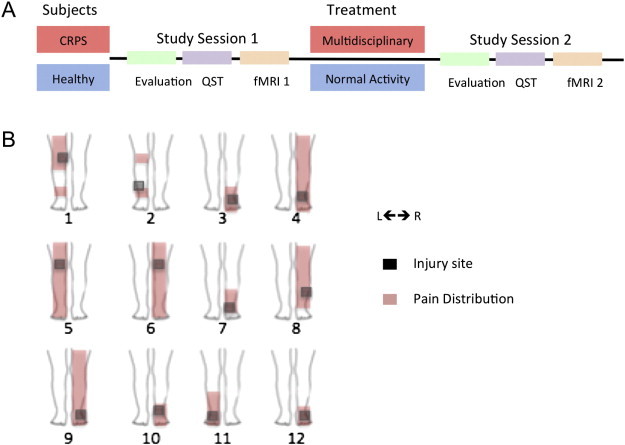
Study design and pain location in CRPS patients. The top panel (A) shows the overall study design for CRPS and control (healthy) subjects involved in the study. Details of the multidisciplinary treatment are noted in the text; patients underwent medical evaluation, quantitative sensory testing (QST), and a functional MRI session. The bottom panel (B) shows the area affected in the lower extremity with the distribution of pain (red) and the area of maximal pain (gray square).
2.2. Procedure
Informed parental consent and participant's assent were obtained at study enrollment. Participants participated in two study sessions, at admission and at discharge from the PPRC for patients and at a matched time interval for controls. No new medications were prescribed during treatment (i.e., each patient remained on the same pharmacological treatment as when they entered into the program). During each study session, participants underwent a focused neurological exam, quantitative sensory testing, and MRI scan (Fig. 1A).
2.3. PPRC interdisciplinary treatment and testing
The rehabilitation program entails intensive daily physical, occupational and psychological therapies 8 h a day, 5 days per week for a typical length of stay of 3 weeks (for details see Logan et al., 2012). Patients received 3–4 h of physical and occupational therapy, families participated actively in the program. Education was provided to the patients' parents and family members. Psychological treatment entailed daily individual and group-based cognitive behavioral therapy. Psychological therapy targets included: (1) teaching a self-management approach to pain, (2) addressing negative thinking and fears about pain, (3) engaging in valued activities and relationships in the presence of pain, and (4) reducing parental attention and protective responses to pain (Logan et al., 2012). A physician and nurse evaluated patients daily to ensure continued appropriateness of treatment (e.g., continued medical stability) and to address acute and/or ongoing medical issues.
2.4. Psychological assessment
CRPS patients completed a battery of psychological measures at admission and discharge from the program. The battery included the Children's Depression Inventory (CDI; Smucker et al., 1986) and the Multidimensional Anxiety Scale for Children (MASC; March et al., 1997) and was administered by trained psychologists. For the CDI and MASC the standardized (T) scores are interpreted as follows: >65 clinically significant symptoms; 60–65 elevated symptoms; below 60 within normal limits.
2.5. Physical and functional assessments
Measurements of physical and functional abilities were collected at admission and discharge of the program. This included the Functional Disability Inventory (FDI; (Walker and Greene, 1991; Claar and Walker, 2006)) and the Lower Extremity Functional Scale (LEFS; (Binkley et al., 1999; Gabel et al., 2012)). For the FDI, scores of 0–12 indicate no to mild disability, 13–29 moderate disability, and greater than 29 indicates severe disability. For the LEFS, scores range from 0 (lowest functioning) to 80 (highest functioning) with a 9-point change considered clinically significant improvement/decrement in functioning.
2.6. Quantitative sensory testing (QST)
Quantitative sensory testing was performed at each study visit. Mechanical (brush) and thermal stimuli were applied on the cutaneous area of the affected lower limb in CRPS patients and their pain scores were recorded on a scale from 0 (no pain) to 10. To determine the level of pain evoked by mechanical allodynia, the skin was brushed with a hand-held soft bristle brush. To determine the cold and heat pain thresholds, the skin was cooled down or warmed up linearly at a slow rate (1 °C/s) using a Medoc Pathway system (Medoc, Haifa, Israel) fitted with a 30 × 30 mm ATS thermode. Participants were instructed to press a button on a patient response unit when pain sensation was perceived, causing the temperature probe to return rapidly to 32 °C. As a safeguard, the temperature limits of the thermode are set at 0 °C and 52 °C. Three cold and three heat trials were administered, with a 10-s inter-trial interval. The cold and heat pain thresholds were calculated by averaging the temperature values obtained in the three trials. An identical procedure was used for controls.
2.7. Statistical analyses
Psychophysical data was analyzed using paired-wise t-tests.
2.8. MRI acquisition and analysis
Participants were scanned on a 3T Siemens Tim Trio MRI scanner using a 12-channel head coil.
2.8.1. Imaging
Two sets of anatomical images were acquired using a magnetization prepared rapid gradient echo (MPRAGE) sequence. Images were acquired in a sagittal plane with a field of view of 256 mm2 [128 1.33-mm-thick slices with an in-plane resolution of 1 mm (256 × 256 voxels)]. Functional: Functional images were acquired utilizing an echo-planar imaging gradient echo sequence with isotropic voxels of 3.5 mm3. Forty-one slices (64 × 64 in-plane resolution) were acquired per volume with TR/TE/Flip Angle = 2.5 s/30 ms/90° with 200 volumes for the resting state scan.
2.8.2. Intrinsic brain networks
To determine brain resting state networks and assess differences between patients and controls as well as pre- and post-treatment effects we utilized a dual regression approach (Filippini et al., 2009; Khalili-Mahani et al., 2012) implemented in FSL (FSL tools). Briefly, the 4 datasets (patients and controls, visits 1 and 2) were first pre-processed (motion correction, spatial smoothing with 5 mm kernel and temporally high pass-filtered with a 100 s time constant) and assessed for quality (excessive motion, severe susceptibility-induced distortions). The preprocessed datasets were concatenated and analyzed with MELODIC (FSL tools) to determine common independent components across the 4 groups. The optimal number of independent components was determined by the software (melodic). For the dual regression analysis, components were used as explanatory variables (EVs) for a generalized linear model approach to model each volume (time point) for each subject. Coefficients were then demeaned and normalized (total amplitude of 1) and assembled as a second EV for each component. Subsequently, each brain was modeled with all the EVs to determine spatial maps that reflect connectivity strength of each voxel with each independent component (Filippini et al., 2009). To assess group differences, the following contrasts were created to determine: 1) Disease effect (Patients visit 1 vs. Controls visit 1): evaluates basal differences between patients and controls; 2) treatment effect (Patients visit 2 vs. Patients visit 1): assesses correlations of differences in brain network connectivity with changes in spontaneous pain following treatment, see below for details of the analysis; (Fig. 2); and 3) residual effect (Patients visit 2 vs. Controls visit 2): determines brain areas that display remaining significant differences from controls. We also investigated if there were differences between visits 1 and 2 for controls due to order effects: Test–retest (Controls visit 1 vs. Controls visit 2).
Fig. 2.
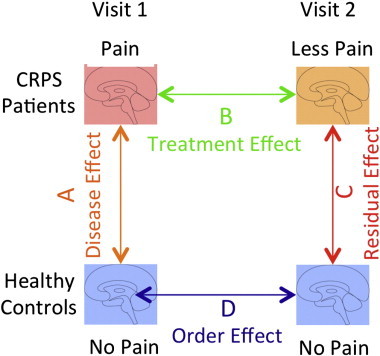
Study questions. The figure depicts the 2 groups (CRPS patients and healthy controls) and the questions we aimed to examine: A: Are there brain changes due to CRPS?; B: Does treatment modulate brain changes?; C: What are the residual effects after treatment?; D: Are there order effects in healthy controls/reproducibility differences?
Following the aggregated ICA analysis, each component was spatially correlated (pears-n correlation) with adult networks as available from FSL (http://www.fmrib.ox.ac.uk/analysis/brainmap+rsns). The dataset with 20 networks was selected and in addition to those listed in Smith et al. (2012); a network that matched the description of structures for the salience network (Seeley et al., 2007) was included. Spatial Pearson correlation coefficients were calculated and a threshold of 0.3 was used to determine significant spatial correspondence between the templates and the components derived for the children in this sample. The component with the highest correlation with a template was identified as the appropriate network.
Treatment effect: Resting state network correlation with spontaneous pain scores. Dual regression was carried out as described above for patients pre- and post-treatment with an additional explanatory variable that consisted of the spontaneous pain scores before and after treatment. This analysis was carried out since patients depicted variable degrees of treatment-concomitant improvement (Fig. 3) and it was thought that a correlation with a behavioral measure would be more appropriate than an imaging-only one. The networks utilized for the comparison, however, where those deduced in the overall ICA analysis described above. We interpreted brain areas with correlated connectivity strength and spontaneous pain intensity as areas associated with pain modulation by treatment. Positive correlation indicated brain areas with increased connectivity with increased pain, while negative correlations would indicate areas hypo-connected in high pain.
Fig. 3.
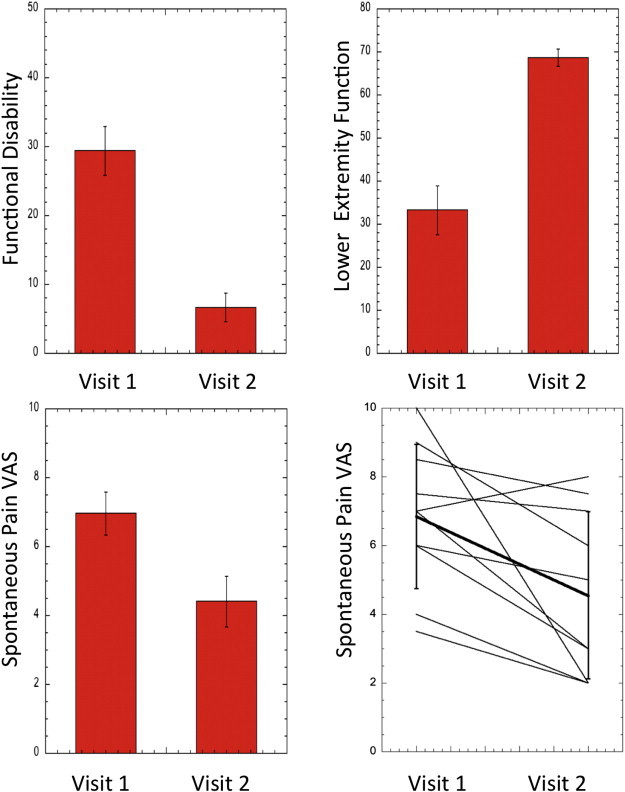
Psychological/psychophysical measures of improvement with treatment. The top panels depict scores for functional disability and lower extremity function changes following treatment, all changes were statistically significant (t-test, p < 0.05). The bottom panels display changes in spontaneous VAS scores for visit 2 vs. visit 1. The line graph depicts individual changes in spontaneous pain in patients in visits 1 and 2; the solid black line represents the average ± standard deviation.
2.8.3. Inference
A generalized false discovery rate (FDR) approach was used to determine statistical thresholds for significance (Pendse et al., 2009). The approach consists of first applying a mixture model analysis: the histogram of the z values is modeled by 3 Gamma distributions (adjusting center and width for each) representing in this case “increased/decreased connectivity” and a null distribution. To determine the threshold for increased connectivity, the decreased connectivity and null classes were joined and used as a “null” for the purpose of determining the threshold using standard FDR. Similarly, to determine the threshold of the decreased connectivity the null and increased connectivity classes were joined. Increased/decreased connectivity thresholded statistical maps were then spatially clustered (minimum cluster size of 7 smoothed native space voxels: 0.9 cm3) to determine brain areas of regional activity with in-house software. After clustering, peak activity within each cluster was referred to a standard MRI atlas (Maldjian et al., 2003) and tabulated. Volumes for whole brain as well as for lobes, subcortical, and cerebellum/brainstem were calculated and plotted as bar graphs.
3. Results
3.1. Participants
Twenty-four participants completed both study sessions (12 CRPS patients (14.1 ± 0.7 yrs (mean ± SEM)) and 12 controls (14.2 ± 0.8 yrs), t(12) = 0.09, p = 0.93) and were included in the final dataset. For the CRPS group, the average duration of pain was 18.9 ± 7.0 months (mean ± SEM) (See Table 1) and the distribution of affected areas appears in Fig. 1B. The healthy controls were tightly matched to the CRPS patients in regard to sex, age, and scanning interval; each group consisted of 9 females and 3 males, 2 females and 1 male were prepubertal.
Table 1.
Demographic and clinical characteristics of the CRPS subjects.
| Subject | Gender | Age | Etiology | Pain location | Pain duration (mo) | VAS1 | VAS2 | Medication | Ethnicity | Pubertal status | Birth history |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | F | 17 | Knee injury | R knee | 2.5 | 7.5 | 7 | AC | wnh | Post | Unremarkable |
| 2 | F | 10 | Twisted ankle | R foot | 18.7 | 9 | 6 | AD | wnh | Pre | Unremarkable |
| 3 | M | 11 | Foot injury | L foot | 8 | 10 | 2 | AC, AD | wnh | Pre | Unremarkable |
| 4 | F | 15 | Crush injury foot | L ankle | 5 | 7 | 8 | AD | wnh | Post | Unremarkable |
| 5 | M | 15 | No known injury | R knee | 12 | 8 | 3 | AC, AD | wnh | Post | Unremarkable |
| 6 | F | 11 | Knee injury | L knee | 6.5 | 6 | 3 | AC, AD | wnh | Pre | Unremarkable |
| 7 | F | 16 | Fractured fibula | L ankle | 48 | 3.5 | 2 | AD | wnh | Post | Unremarkable |
| 8 | F | 17 | Foot/knee injury | L ankle | 19 | 4 | 2 | AD | wnh | Post | NICU for 2 weeks post-birth |
| 9 | F | 14 | Sprained ankle | L foot | 2.5 | 8.5 | 7.5 | AC | wnh | Post | Unremarkable |
| 10 | M | 13 | Post-surgery | L ankle | 6 | 6 | 2 | AC | wnh | Pre | Vaginal delivery to term; clavicle fracture at birth |
| 11 | F | 17 | Twisted ankle | R foot | 85 | 6 | 5 | AD | wnh | Post | Not mentioned |
| 12 | F | 13 | Ankle sprain | L foot | 13 | 7 | 3 | AC | wnh | Post | 35-week preterm delivery |
| Mean ± SEM | 14.1 0.72 | 18.9 (7.0) | 7.0 0.56 | 4.4 0.67 |
R = right; L = left. VAS1 (2) = pain rating at visit 1 (2). AC, anticonvulsants (pregabalin, gabapentin); AD, anti-depressants (amitriptyline, duloxetine).
3.2. Psychological and psychophysical assessments
3.2.1. Depressive and anxiety symptoms
Although average scores on the CDI were within normal limits, there was a decrease in scores from admission to discharge for the total CDI depressive symptom score (t(7) = 1.88, p = 0.10) and the anhedonia subscale (t(7) = 2.77, p = 0.03). CRPS patients scored within normal limits on all subscales of the MASC at admission. MASC scores decreased with the Anxiety Disorders Index statistically significant (t(7) = 2.59, p = 0.04) (see Table 2 for further detail).
Table 2.
Depression and anxiety measures in the CRPS patient sample. The CDI total score, anhedonia score, and the Anxiety Disorders Index decreased significantly from intake to discharge (paired-sample t-tests, two-tailed). NS, non-significant (p > 0.1).
| Test | Mean T visit 1 (SEM) | Mean T visit 2 (SEM) | Statistics |
|---|---|---|---|
| CDI total | 58.27 (4.27) | 49.60 (4.19) | t(8) = 2.337, p = 0.048 |
| Negative mood | 56.36 (4.29) | 50.50 (4.50) | NS |
| Interpersonal problems | 51.00 (3.26) | 50.20 (2.67) | NS |
| Ineffectiveness | 56.27 (4.25) | 47.20 (3.04) | t(8) = 2.063, p = 0.073 |
| Anhedonia | 60.00 (3.39) | 51.40 (3.29) | t(8) = 3.125, p = 0.014 |
| Negative self esteem | 51.82 (3.48) | 48.50 (4.37) | NS |
| MASC total | 50.45 (3.73) | 43.50 (3.69) | NS |
| Inconsistency Index | 5.36 (0.53) | 4.40 (0.99) | NS |
| Physical symptoms | 50.27 (3.20) | 42.90 (2.74) | NS |
| Harm avoidance | 47.18 (2.34) | 43.50 (2.64) | NS |
| Social anxiety | 53.00 (3.29) | 46.30 (3.36) | t(8) = 1.974, p = 0.084 |
| Separation/panic | 54.73 (3.14) | 49.80 (3.27) | NS |
| Anxiety Disorders Index | 53.55 (2.95) | 43.80 (2.41) | t(8) = 3.06, p = 0.016 |
R = right; L = left. VAS1 (2) = pain rating at visit 1 (2). AC, anticonvulsants (pregabalin, gabapentin); AD, anti-depressants (amitriptyline, duloxetine).
3.2.2. Physical and functional abilities
At admission, FDI scores were at the upper limit of moderate disability (M = 29.4, SD = 11.2) and significantly decreased to mild disability (M = 6.67, SD = 6.67) at discharge (t(8) = 8.84, p < 0.00) (Fig. 3). Lower extremity functional scale (LEFS) scores showed a similarly dramatic improvement with a clinically significant increase in scores with a mean score of 33.3 (SD = 18.1) at admission to 68.6 (SD = 6.5) at discharge (t(8) = 5.19, p < 0.00; an increase in 9 points is considered clinically significant improvement and we observed a change of 35.3 — almost 4-fold) (Fig. 3).
3.2.3. Temperature thresholds
Pain threshold data is missing for one CRPS subject due to technical issues related to equipment not recording temperature values. Pre-treatment patients displayed similar heat pain threshold than controls (41.77 ± 1.20 °C vs. 40.47 ± 3.19 °C, t(11) = 0.41, p = 0.41). Following treatment, patients displayed and increased (not significant) heat pain threshold compared to controls (44.18 ± 1.03 °C vs. 39.16 ± 2.87 °C, t(11) = 1.77, p = 0.09). For cold; patients had a significant difference pre-treatment with controls (21.18 ± 3.23 °C vs. 11.36 ± 2.64 °C, t(11) = 2.29, p = 0.03). Post-treatment; the difference between patients and controls was statistically not significant (14.08 ± 3.64 °C vs. 14.77 ± 3.00 °C, t(11) = 0.14, p = 0.88). Brushing of affected area produced a painful response in patients but not in controls (0–10 scale) (7.05 ± 0.78 vs. 0.23 ± 0.18, t(11) = 8.14, p < 0.0001).
3.2.4. Spontaneous pain ratings
Spontaneous pain decreased significantly between visits 1 and 2 (visit 1: 7.0 ± 2.0; visit 2: 4.41 ± 0.7, t(9) = –2.48, p = 0.025).
3.3. Intrinsic brain networks
Five scans displayed excessive motion (>3 mm) resulting in the following group numbers (Controls visit 1 N = 12, Controls visit 2 N = 10, Patients visit 1 N = 11, Patients visit 2 N = 10). For the treatment effect comparison (Patients visit 1 vs. visit 2) one patient visit 1 scan was eliminated to perform a paired-analysis.
3.3.1. Network identification
To identify specific networks the following approach was used. An independent component analysis (that optimized the number of components) of all 4 groups resulted in 49 independent components. Out of the 49 components we were able to identify all of the networks previously described for healthy adults (Beckmann et al., 2005; Salvador et al., 2005; Damoiseaux et al., 2006; De Luca et al., 2006; van den Heuvel et al., 2008), (Smith et al., 2009) and children (Thomason et al., 2011): We identified visual networks (medial, occipital, and lateral), default mode, cerebellar, sensorimotor, auditory, central executive, fronto-parietal (left and right), and salience networks (Fig. 4). Pearson correlation coefficients for the healthy children network with the correspondent adult network were above 0.3. Some networks displayed small differences between adults and children: the default mode network in children had less perigenual cingulate and parietal lobe involvement, the cerebellum network in children does not involve the brainstem as in adults. The occipital visual network in children does not include the thalamus. The other networks were highly congruent.
Fig. 4.
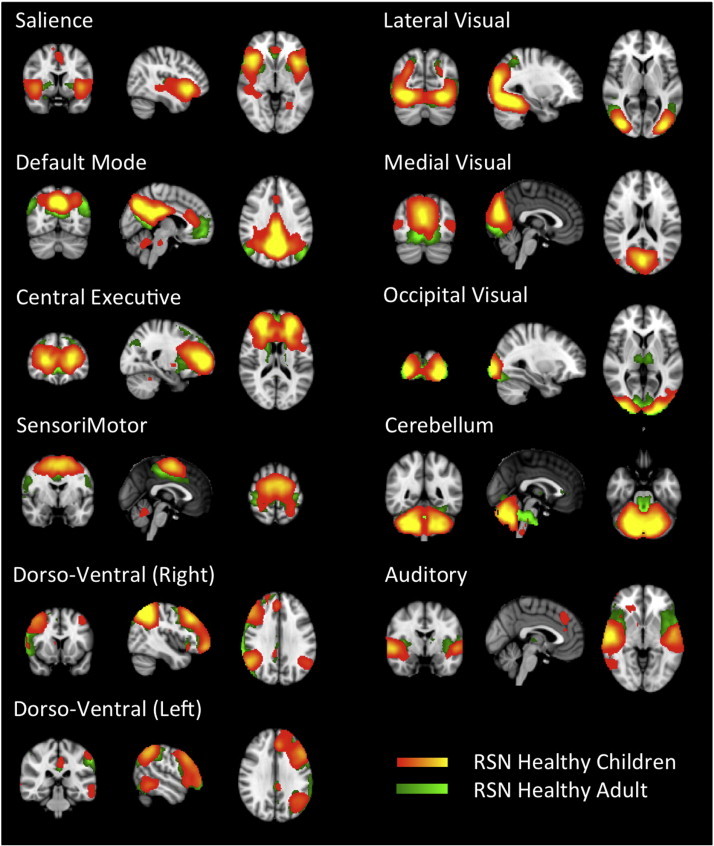
Healthy children resting state networks. The figure depicts resting state networks identified in healthy children (red–yellow) with the corresponding adult ones (green–light green). The correspondence was high but some minor differences were observed, for instance the default mode network in children had a reduce involvement of the perigenual cingulate as well as superior parietal lobe. The cerebellum network in children seemed to be restricted to the structure while the adult one extended into the brainstem. See text for more details.
3.3.2. Disease effect: differences between CRPS patients and healthy controls (visit 1)
Figs. 5 and 6 show the significant differences in brain network connectivities due to disease effects. Details of activation regions are defined in Table 3 (disease effect). In all cases significant differences were observed with red indicating CRPS > Controls and blue indicating CRPS < Controls. The most prominent differences are summarized below.
Fig. 5.
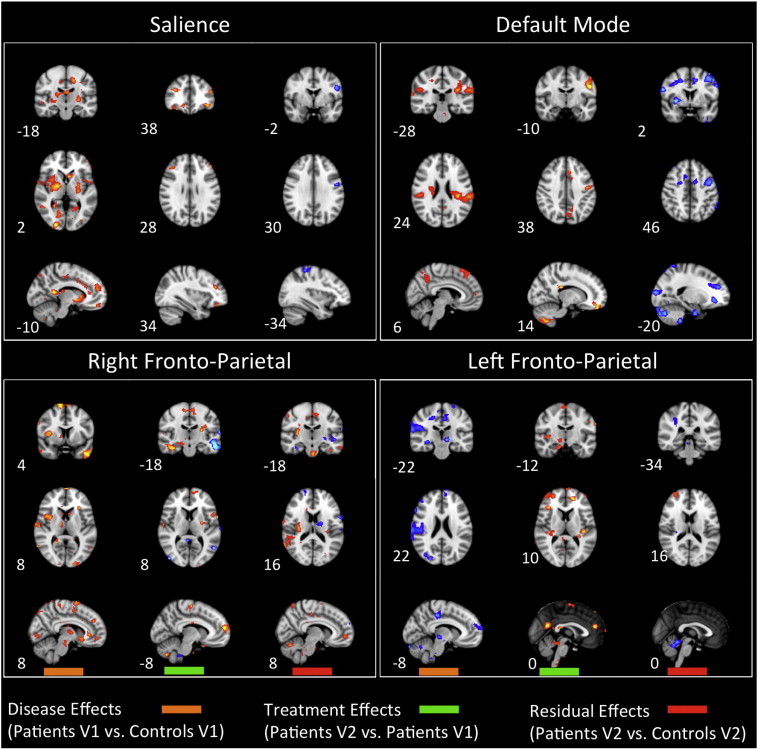
Disease, treatment, and residual effects on networks: salience, default mode, and fronto-parietal network spatial maps: The graphs summarize statistically significant changes in connectivity measured between patients and controls at visit 1 (disease effect), between patients at visit 2 and visit 1 as determined through their correlation with spontaneous VAS scores (treatment effect), and patients vs. controls at visit 2 (residual effect). Prominent changes can be observed visually. See text for further details. Numbers refer to the standard MNI Atlas coordinates.
Fig. 6.
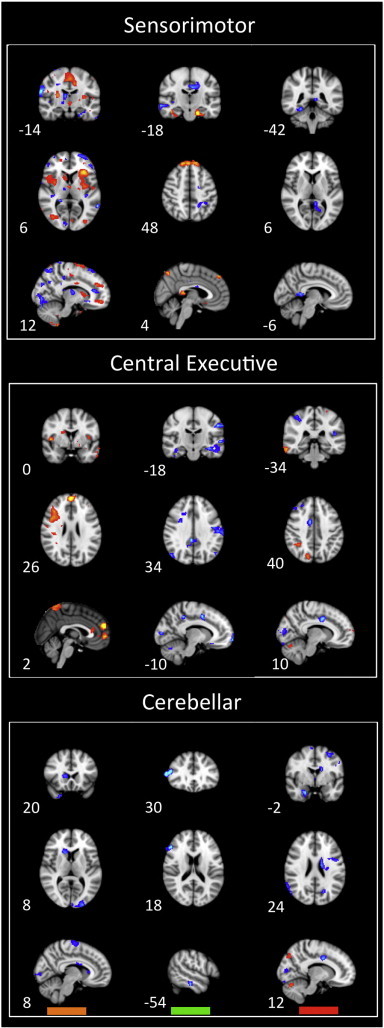
Disease, treatment, and residual effects on networks: sensorimotor, central executive, and cerebellar network spatial maps: The graphs summarize statistically significant changes in connectivity measured between patients and controls at visit 1 (disease effect), between patients at visit 2 and visit 1 as determined through their correlation with spontaneous VAS scores (treatment effect), and patients vs. controls at visit 2 (residual effect). Prominent changes can be observed visually. See text for further details. Numbers refer to the standard MNI Atlas coordinates.
Table 3.
Disease effects: The table indicates brain areas of significant increased or decreased connectivity with the listed networks for CRPS Patients visit 1 vs. Controls visit 1. Coordinates and max statistical value (z-stat) are given for peak activity as well as volume (Vol) of each cluster of activity. See Materials and methods section for details.
| Brain region | Lat. | z-stat | X (mm) | Y (mm) | Z (mm) | Vol (cm) | |
|---|---|---|---|---|---|---|---|
| Salience | |||||||
| Positive (Patients V1 > Controls V1) | Cortical | ||||||
| Frontal | |||||||
| Superior | L | 3.31 | −26 | 58 | 24 | 1.56 | |
| L | 3.52 | −26 | 50 | 20 | 4.54 | ||
| L | 3.29 | −14 | 50 | 20 | 7.26 | ||
| L | 3.49 | −22 | 6 | 64 | 4.10 | ||
| Rectus | L | 3.14 | −6 | 54 | −20 | 1.70 | |
| Superior medial | L | 2.96 | −6 | 50 | 32 | 3.71 | |
| Inferior triangular | L | 3.21 | −38 | 30 | 16 | 8.23 | |
| L | 3.01 | −42 | 14 | 24 | 3.46 | ||
| Supp_Motor_Area | R | 3.82 | 14 | 14 | 64 | 4.62 | |
| R | 3.06 | 10 | −2 | 60 | 1.46 | ||
| R | 3.17 | 10 | −6 | 68 | 1.20 | ||
| Olfactory | L | 3.48 | −2 | 14 | −4 | 3.07 | |
| Middle orbital | R | 3.06 | 42 | 14 | 56 | 6.15 | |
| Middle | L | 3.04 | −30 | 2 | 60 | 1.78 | |
| Precentral | L | 3.01 | −34 | −14 | 40 | 6.20 | |
| Parietal | |||||||
| Supramarginal | R | 3.33 | 50 | −26 | 24 | 3.56 | |
| Inferior | R | 3.09 | 50 | −38 | 48 | 4.56 | |
| Precuneus | L | 4.37 | −10 | −46 | 12 | 9.52 | |
| R | 3.67 | 18 | −50 | 16 | 4.14 | ||
| Superior | R | 3.49 | 14 | −50 | 64 | 4.03 | |
| L | 3.08 | −26 | −62 | 44 | 1.48 | ||
| L | 4.65 | −30 | −66 | 56 | 4.03 | ||
| Angular | R | 3.81 | 46 | −62 | 52 | 9.15 | |
| Occipital | |||||||
| Rolandic operculum | R | 4.24 | 42 | −6 | 16 | 8.96 | |
| Calcarine | R | 3.25 | 18 | −74 | 4 | 3.70 | |
| R | 4.53 | 22 | −94 | 0 | 2.46 | ||
| Superior | L | 3.00 | −22 | −86 | 40 | 1.93 | |
| Middle | L | 2.96 | −18 | −90 | −8 | 4.34 | |
| Inferior | R | 4.67 | 26 | −90 | −4 | 3.86 | |
| Temporal | |||||||
| Pole superior | R | 3.90 | 54 | 10 | −8 | 12.59 | |
| Superior | R | 3.11 | 62 | 2 | −4 | 1.97 | |
| Fusiform | R | 3.38 | 34 | −2 | −36 | 1.13 | |
| Middle | R | 3.02 | 50 | −26 | −8 | 6.57 | |
| R | 3.48 | 46 | −50 | 20 | 7.41 | ||
| R | 3.20 | 54 | −54 | 0 | 4.91 | ||
| Inferior | R | 3.39 | 42 | −6 | −40 | 2.50 | |
| R | 3.40 | 50 | −42 | −20 | 1.54 | ||
| R | 3.11 | 46 | −42 | −24 | 1.79 | ||
| Lingual | R | 3.49 | 18 | −42 | −12 | 1.72 | |
| L | 4.47 | −22 | −50 | −8 | 14.27 | ||
| L | 2.99 | −14 | −58 | 0 | 2.91 | ||
| R | 3.01 | 18 | −66 | 0 | 2.50 | ||
| Fusiform | L | 3.02 | −26 | −78 | −12 | 3.26 | |
| L | 3.13 | −22 | −82 | −12 | 4.28 | ||
| Cingulum | |||||||
| Anterior | L | 3.20 | −6 | 22 | 28 | 10.82 | |
| Parahippocampus | |||||||
| Parahippocampal | R | 3.43 | 30 | 10 | −32 | 7.94 | |
| Sub-cortical | |||||||
| Putamen | R | 3.96 | 26 | 14 | 12 | 4.51 | |
| R | 3.19 | 30 | 6 | 4 | 2.62 | ||
| L | 3.97 | −26 | −2 | 8 | 22.82 | ||
| Caudate | L | 3.84 | −6 | 14 | 0 | 2.83 | |
| Pallidum | L | 3.54 | −10 | 6 | −4 | 2.57 | |
| R | 5.31 | 26 | −10 | −4 | 8.22 | ||
| Hypothalamus | 3.05 | 6 | −10 | −4 | 1.86 | ||
| Brainstem/cerebellum | |||||||
| Cerebellum 3 | R | 3.79 | 14 | −38 | −24 | 6.40 | |
| Cerebellum 6 | L | 3.47 | −30 | −42 | −28 | 6.66 | |
| Vermis 3 | L | 3.28 | −2 | −46 | −16 | 1.98 | |
| Cerebellum Crus 1 | R | 3.12 | 26 | −66 | −36 | 2.92 | |
| Cerebellum 8 | L | 2.96 | −10 | −66 | −44 | 10.19 | |
| Negative (Patients V1 < Controls V1) | No statistically significant differences | ||||||
| Central executive network (CEN) | |||||||
| Positive (Patients V1 > Controls V1) | Cortical | ||||||
| Frontal | |||||||
| Superior orbital | L | 1.99 | −22 | 66 | −4 | 2.62 | |
| R | 2.21 | 26 | 26 | 52 | 1.00 | ||
| Middle orbital | R | 2.05 | 42 | 54 | 4 | 4.65 | |
| Inferior orbital | L | 3.70 | −18 | 14 | −24 | 3.35 | |
| Superior medial | L | 3.82 | −2 | 58 | 24 | 8.26 | |
| R | 2.26 | 18 | 38 | −16 | 2.48 | ||
| R | 2.91 | 18 | 22 | −20 | 3.98 | ||
| Frontal pole | 2.81 | 2 | 58 | 0 | 15.29 | ||
| 2.19 | −26 | 42 | −12 | 3.29 | |||
| Middle | L | 2.04 | −42 | 50 | 4 | 1.75 | |
| L | 2.03 | −38 | 46 | 4 | 1.56 | ||
| L | 2.33 | −30 | 22 | 40 | 2.66 | ||
| L | 2.34 | −22 | 18 | 44 | 1.01 | ||
| Superior | L | 2.19 | −14 | 38 | 36 | 4.50 | |
| Inferior triangular | R | 2.27 | 46 | 38 | 8 | 2.45 | |
| R | 2.84 | 38 | 26 | 28 | 6.58 | ||
| Inferior operculum | R | 2.93 | 42 | 10 | 28 | 4.51 | |
| Precentral | L | 2.08 | −50 | 6 | 48 | 2.95 | |
| Parietal | |||||||
| Postcentral | R | 2.05 | 58 | −6 | 28 | 1.10 | |
| R | 2.60 | 22 | −34 | 68 | 5.02 | ||
| L | 2.36 | −18 | −38 | 76 | 3.07 | ||
| Inferior | L | 2.36 | −50 | −42 | 56 | 1.93 | |
| Precuneus | R | 2.39 | 10 | −46 | 56 | 1.19 | |
| L | 2.81 | −2 | −46 | 68 | 5.52 | ||
| Occipital | |||||||
| Inferior | L | 2.34 | −46 | −78 | −4 | 1.98 | |
| Superior | L | 2.77 | −14 | −82 | 40 | 1.82 | |
| Temporal | |||||||
| Pole superior | L | 2.21 | −50 | 10 | −24 | 4.24 | |
| Middle | L | 2.18 | −62 | −2 | −16 | 4.58 | |
| R | 2.66 | 62 | −18 | −8 | 1.87 | ||
| L | 2.22 | −50 | −26 | −16 | 1.68 | ||
| R | 2.15 | 66 | −38 | −8 | 2.63 | ||
| L | 3.31 | −62 | −50 | −12 | 1.75 | ||
| Inferior | L | 2.02 | −42 | −6 | −40 | 1.72 | |
| R | 2.67 | 62 | −34 | −24 | 1.53 | ||
| L | 3.80 | −58 | −54 | −16 | 1.49 | ||
| L | 2.45 | −50 | −62 | −8 | 1.39 | ||
| Heschl | R | 2.69 | 34 | −30 | 12 | 3.04 | |
| Fusiform | L | 2.76 | −34 | −22 | −28 | 1.08 | |
| Lingual | R | 3.16 | 14 | −42 | −4 | 2.30 | |
| L | 2.51 | −14 | −90 | −16 | 2.53 | ||
| Cingulum | |||||||
| Anterior | R | 2.12 | 10 | 34 | 12 | 1.05 | |
| R | 2.16 | 14 | 30 | 16 | 3.36 | ||
| R | 2.01 | 2 | 30 | 12 | 3.13 | ||
| Insula | |||||||
| Anterior | L | 2.03 | −38 | 22 | 4 | 2.08 | |
| Posterior | R | 3.09 | 46 | −2 | 4 | 3.70 | |
| L | 2.86 | −38 | −6 | 8 | 2.86 | ||
| Brainstem/cerebellum | |||||||
| Cerebellum Crus 2 | R | 2.33 | 46 | −54 | −48 | 1.29 | |
| Cerebellum 6 | L | 2.35 | −34 | −54 | −24 | 1.60 | |
| L | 2.80 | −22 | −62 | −24 | 2.13 | ||
| Cerebellum Crus 1 | R | 2.10 | 34 | −66 | −28 | 1.17 | |
| L | 2.06 | −42 | −74 | −36 | 2.04 | ||
| L | 3.35 | −18 | −82 | −28 | 1.98 | ||
| Cerebellum 7b | L | 2.42 | −14 | −78 | −44 | 1.48 | |
| Brainstem | L | 2.05 | −6 | −30 | −12 | 1.78 | |
| Negative (Patients V1 < Controls V1) | No statistically significant differences. | ||||||
| Default mode network | |||||||
| Positive (Patients V1 > Controls V1) | Cortical | ||||||
| Frontal | |||||||
| Superior | R | 3.45 | 10 | 38 | 56 | 1.02 | |
| R | 3.37 | 10 | 26 | 60 | 1.12 | ||
| Superior medial | L | 3.09 | −10 | 58 | 0 | 1.10 | |
| Parietal | |||||||
| Postcentral | L | 3.10 | −42 | −22 | 44 | 1.57 | |
| Supramarginal | L | 3.75 | −58 | −30 | 24 | 1.62 | |
| L | 3.65 | −54 | −42 | 32 | 1.19 | ||
| Precuneus | R | 2.75 | 6 | −54 | 40 | 1.58 | |
| Superior | L | 2.92 | −18 | −74 | 44 | 1.02 | |
| Temporal | |||||||
| Operculum | R | 3.20 | 46 | −26 | 24 | 1.37 | |
| L | 4.20 | −34 | −30 | 24 | 4.83 | ||
| Negative (Patients V1 < Controls V1) | No statistically significant differences | ||||||
| Sensorimotor network | |||||||
| Positive differences (Patients visit 1 > Controls visit 1) | Cortical | ||||||
| Frontal | |||||||
| Rectus | R | 2.35 | 2 | 58 | −16 | 2.68 | |
| Superior medial | L | 3.32 | 2 | 58 | 24 | 3.47 | |
| L | 2.44 | −2 | 46 | 36 | 6.54 | ||
| Middle | L | 3.14 | −34 | 46 | 0 | 2.10 | |
| R | 2.86 | 2 | 42 | −4 | 4.51 | ||
| L | 2.82 | −30 | 26 | 44 | 6.15 | ||
| L | 2.50 | −26 | 26 | 36 | 1.86 | ||
| L | 2.43 | −38 | 14 | 52 | 6.43 | ||
| Superior orbital | R | 3.49 | 18 | 42 | 28 | 3.84 | |
| R | 2.54 | 30 | −2 | 60 | 9.04 | ||
| Superior medial | L | 3.28 | −2 | 26 | 56 | 10.74 | |
| Inferior triangular | R | 3.18 | 42 | 18 | 28 | 7.02 | |
| Inferior operculum | R | 2.35 | 46 | 14 | 12 | 7.70 | |
| Supp_Motor_Area | R | 2.88 | 10 | 2 | 72 | 7.85 | |
| L | 2.86 | −2 | −14 | 52 | 3.49 | ||
| R | 3.01 | 6 | −18 | 52 | 1.99 | ||
| R | 2.80 | 2 | −18 | 56 | 6.61 | ||
| Parietal | |||||||
| Postcentral | L | 2.42 | −50 | −6 | 36 | 8.55 | |
| R | 3.18 | 34 | −30 | 64 | 8.16 | ||
| Supramarginal | L | 2.59 | −50 | −26 | 16 | 2.49 | |
| R | 2.33 | 54 | −30 | 36 | 4.17 | ||
| Inferior | L | 3.57 | −46 | −38 | 36 | 4.70 | |
| Superior | R | 2.54 | 30 | −70 | 48 | 1.73 | |
| Occipital | |||||||
| Middle | R | 3.15 | 38 | −78 | 0 | 2.48 | |
| Inferior | R | 2.68 | 34 | −78 | −8 | 3.75 | |
| Cuneus | L | 2.99 | −2 | −82 | 20 | 6.02 | |
| Calcarine | L | 2.36 | −6 | −82 | 4 | 2.18 | |
| Temporal | |||||||
| Pole Superior | L | 3.39 | −34 | 6 | −24 | 4.38 | |
| Superior | L | 2.81 | −46 | −2 | −4 | 2.10 | |
| Middle | R | 3.27 | 54 | −42 | −8 | 1.68 | |
| R | 3.56 | 54 | −46 | −4 | 4.17 | ||
| L | 2.51 | −38 | −66 | 12 | 1.75 | ||
| Inferior | R | 3.63 | 62 | −38 | −20 | 2.34 | |
| R | 3.46 | 58 | −46 | −12 | 1.48 | ||
| Cingulum | |||||||
| Middle | R | 2.70 | 6 | −14 | 48 | 2.76 | |
| L | 2.76 | −10 | −14 | 40 | 6.56 | ||
| Insula | |||||||
| Anterior | L | 3.64 | −30 | 26 | 4 | 14.58 | |
| R | 2.74 | 34 | 2 | 8 | 1.29 | ||
| Posterior | R | 2.36 | 42 | −2 | −12 | 3.10 | |
| L | 3.25 | −34 | −6 | 8 | 9.04 | ||
| Parahippocampus | |||||||
| Parahippocampal | L | 2.78 | −18 | −2 | −24 | 3.61 | |
| L | 3.44 | −26 | −38 | −8 | 6.68 | ||
| Sub-cortical | |||||||
| Caudate | R | 2.48 | 10 | 22 | 4 | 5.37 | |
| Putamen | R | 3.00 | 30 | 2 | 12 | 1.96 | |
| Hypothalamus | R | 3.37 | 2 | −10 | 0 | 1.42 | |
| Hippocampus | L | 2.97 | −22 | −26 | −8 | 2.27 | |
| Brainstem/cerebellum | |||||||
| Cerebellum 4 5 | L | 2.52 | −22 | −38 | −32 | 4.09 | |
| Cerebellum 8 | R | 3.33 | 14 | −46 | −60 | 1.54 | |
| Cerebellum 6 | R | 2.63 | 38 | −46 | −32 | 2.38 | |
| Cerebellum 9 | L | 2.43 | −10 | −50 | −40 | 2.50 | |
| Cerebellum Crus 1 | L | 2.40 | −26 | −82 | −32 | 1.90 | |
| L | 2.52 | −10 | −86 | −20 | 1.86 | ||
| Negative differences (Patients visit 1 < Controls visit 1) | Cortical | ||||||
| Frontal | |||||||
| Superior | L | −2.58 | −22 | 66 | 12 | 3.10 | |
| L | −2.29 | −34 | 58 | 0 | 1.69 | ||
| Superior medial | L | −2.87 | −10 | 42 | 52 | 1.91 | |
| L | −2.81 | −10 | 34 | 56 | 4.03 | ||
| Superior orbital | R | −2.89 | 18 | 22 | 60 | 3.14 | |
| Middle orbital | R | −3.26 | 38 | 34 | 16 | 7.89 | |
| Inferior orbital | R | −3.09 | 46 | 42 | −16 | 3.26 | |
| R | −2.92 | 46 | 34 | −8 | 2.42 | ||
| Middle | L | −2.78 | −34 | 58 | 16 | 3.82 | |
| L | −2.29 | −34 | 38 | 16 | 1.01 | ||
| Inferior_Triangular | L | −2.63 | −42 | 46 | 12 | 1.92 | |
| R | −2.31 | 50 | 42 | 4 | 1.21 | ||
| Inferior_Triangular | R | −2.46 | 50 | 34 | 24 | 3.24 | |
| L | −2.86 | −54 | 26 | 0 | 3.70 | ||
| L | −2.24 | −50 | 26 | 24 | 3.90 | ||
| Inferior operculum | R | −2.76 | 58 | 14 | 20 | 1.64 | |
| Precentral | R | −2.56 | 62 | 2 | 24 | 1.69 | |
| Parietal | |||||||
| Postcentral | R | −3.62 | 66 | −14 | 24 | 6.50 | |
| R | −2.99 | 14 | −38 | 76 | 4.37 | ||
| Inferior | L | −2.33 | −54 | −34 | 52 | 4.14 | |
| L | −2.36 | −46 | −50 | 48 | 2.12 | ||
| Precuneus | R | −2.72 | 14 | −62 | 36 | 2.38 | |
| R | −2.59 | 10 | −62 | 60 | 3.08 | ||
| L | −2.46 | −6 | −70 | 36 | 2.98 | ||
| Angular | L | −2.35 | −54 | −62 | 40 | 6.66 | |
| L | −2.64 | −42 | −70 | 48 | 2.92 | ||
| Occipital | |||||||
| Cuneus | R | −2.64 | 22 | −62 | 20 | 3.37 | |
| R | −2.76 | 14 | −78 | 32 | 2.50 | ||
| Inferior | R | −2.78 | 46 | −66 | −16 | 3.68 | |
| R | −2.75 | 34 | −90 | −12 | 1.97 | ||
| Superior | L | −2.60 | −22 | −74 | 24 | 1.97 | |
| R | −2.67 | 26 | −82 | 20 | 1.27 | ||
| Middle | R | −2.53 | 30 | −86 | 32 | 3.18 | |
| Calcarine | R | −2.35 | 10 | −90 | 4 | 2.19 | |
| Temporal | |||||||
| Pole middle | R | −3.28 | 38 | 22 | −36 | 1.71 | |
| R | −3.03 | 30 | 6 | −36 | 10.88 | ||
| Superior | R | −2.31 | 66 | −18 | 12 | 2.38 | |
| L | −2.62 | −42 | −42 | 16 | 1.82 | ||
| Heschl | L | −2.89 | −38 | −22 | 12 | 2.96 | |
| Fusiform | L | −2.77 | −34 | −22 | −28 | 1.44 | |
| Middle | L | −3.88 | −62 | −50 | 4 | 2.41 | |
| Lingual | R | −2.54 | 10 | −82 | −4 | 3.50 | |
| Cingulum | |||||||
| Anterior | L | −2.65 | 2 | 38 | 16 | 8.39 | |
| L | −2.45 | −2 | 30 | 20 | 1.19 | ||
| R | −2.61 | 2 | 2 | 28 | 2.30 | ||
| Middle | R | −2.46 | 2 | 18 | 32 | 1.87 | |
| Posterior | R | −2.28 | 10 | −38 | 28 | 2.27 | |
| Parahippocampus | |||||||
| Parahippocampal | L | −2.67 | −26 | −18 | −28 | 1.09 | |
| R | −2.92 | 30 | −22 | −28 | 1.48 | ||
| L | −2.73 | −30 | −26 | −24 | 1.40 | ||
| Sub-cortical | |||||||
| Hypothalamus | L | −2.30 | −2 | −2 | −8 | 5.14 | |
| Thalamus | R | −2.65 | 10 | −10 | 12 | 3.24 | |
| R | −2.67 | 18 | −18 | 16 | 1.74 | ||
| Caudate | L | −2.90 | −14 | −10 | 20 | 1.55 | |
| Brainstem/cerebellum | |||||||
| Cerebellum 8 | L | −2.44 | −42 | −54 | −52 | 5.07 | |
| Cerebellum Crus 2 | L | −2.44 | −42 | −70 | −48 | 3.08 | |
| Cerebellum Crus 1 | L | −3.37 | −46 | −70 | −28 | 5.42 | |
| R | −2.42 | 38 | −78 | −32 | 2.50 | ||
| R | −3.18 | 18 | −82 | −24 | 2.94 | ||
| Cerebellum | |||||||
| Positive (Patients V1 > Controls V1) | No statistically significant differences | ||||||
| Negative (Patients V1 < Controls V1) | Cortical | ||||||
| Frontal | |||||||
| Supp_Motor_Area | R | 2.49 | 10 | −10 | 68 | 1.45 | |
| Occipital | |||||||
| Superior | L | 2.47 | −22 | −82 | 28 | 1.46 | |
| Middle | L | 3.05 | −26 | −98 | 8 | 3.30 | |
| Calcarine | L | 3.23 | −6 | −98 | 0 | 1.54 | |
| Sub-cortical | |||||||
| Caudate | R | 2.59 | 14 | 6 | 20 | 1.15 | |
| L | 2.33 | −14 | 6 | 20 | 1.28 | ||
| Brainstem/cerebellum | |||||||
| Cerebellum Crus 1 | R | 2.56 | 50 | −62 | −28 | 1.31 | |
| Fronto-parietal network (right) | |||||||
| Positive (Patients V1 > Controls V1) | Cortical | ||||||
| Frontal | |||||||
| Superior orbital | L | 2.72 | −18 | 50 | −12 | 5.05 | |
| R | 2.91 | 18 | 42 | 32 | 6.47 | ||
| Middle orbital | R | 3.24 | 38 | 34 | 36 | 4.40 | |
| R | 3.53 | 34 | 30 | 40 | 3.80 | ||
| Inferior orbital | L | 3.07 | −22 | 34 | −12 | 1.88 | |
| L | 2.62 | −42 | 34 | −12 | 2.10 | ||
| Superior | L | 2.34 | −22 | 46 | 36 | 5.96 | |
| Middle | R | 2.38 | 6 | 50 | −8 | 2.88 | |
| L | 2.58 | −42 | 34 | 32 | 1.02 | ||
| L | 2.39 | −38 | 30 | 44 | 2.68 | ||
| Inferior triangular | R | 2.37 | 50 | 30 | 16 | 5.66 | |
| Olfactory | R | 2.54 | 6 | 18 | −8 | 9.18 | |
| Inferior operculum | L | 2.66 | −46 | 14 | 32 | 5.52 | |
| Supp. motor area | L | 3.16 | −6 | 10 | 68 | 1.32 | |
| R | 2.56 | 10 | −2 | 56 | 5.39 | ||
| L | 2.47 | −10 | −14 | 52 | 2.14 | ||
| Precentral | R | 2.79 | 42 | −2 | 40 | 6.53 | |
| L | 3.01 | −38 | −6 | 56 | 5.55 | ||
| L | 2.78 | −46 | −6 | 52 | 2.72 | ||
| Paracentral lobule | R | 2.64 | 10 | −34 | 68 | 10.97 | |
| Parietal | |||||||
| Supramarginal | R | 2.62 | 58 | −26 | 28 | 3.94 | |
| R | 2.71 | 46 | −42 | 36 | 6.48 | ||
| Postcentral | R | 2.52 | 34 | −30 | 60 | 1.07 | |
| R | 2.90 | 30 | −34 | 64 | 1.55 | ||
| R | 2.55 | 34 | −34 | 56 | 2.24 | ||
| Inferior | L | 2.37 | −30 | −58 | 48 | 6.63 | |
| Occipital | |||||||
| Rolandic operculum | R | 2.76 | 58 | −14 | 12 | 4.52 | |
| Middle | L | 2.37 | −26 | −62 | 36 | 1.85 | |
| L | 3.09 | −26 | −78 | 40 | 7.58 | ||
| L | 2.91 | −18 | −94 | 4 | 2.23 | ||
| L | 2.63 | −18 | −98 | 8 | 1.35 | ||
| Calcarine | R | 2.46 | 18 | −90 | 0 | 1.44 | |
| L | 2.48 | 2 | −90 | 0 | 5.72 | ||
| R | 2.53 | 18 | −98 | 4 | 2.22 | ||
| Temporal | |||||||
| Pole middle | R | 2.89 | 42 | 14 | −36 | 4.10 | |
| Superior | R | 2.38 | 62 | −10 | 0 | 3.90 | |
| R | 2.72 | 50 | −38 | 20 | 5.74 | ||
| Middle | L | 3.51 | −46 | 2 | −32 | 12.31 | |
| R | 2.35 | 54 | −22 | −16 | 2.41 | ||
| R | 3.21 | 54 | −50 | 20 | 1.13 | ||
| R | 3.66 | 62 | −54 | 16 | 4.47 | ||
| Inferior | L | 2.73 | −54 | −6 | −32 | 2.04 | |
| L | 3.38 | −38 | −14 | −36 | 3.45 | ||
| L | 3.83 | −42 | −46 | −16 | 2.47 | ||
| L | 3.65 | −46 | −46 | −12 | 3.75 | ||
| L | 2.52 | −54 | −54 | −20 | 3.90 | ||
| Lingual | L | 2.63 | −10 | −86 | −4 | 3.22 | |
| Cingulum | |||||||
| Anterior | R | 2.86 | 10 | 34 | 0 | 2.19 | |
| Middle | R | 2.44 | 10 | 14 | 40 | 1.96 | |
| Insula | |||||||
| Anterior | L | 3.10 | −30 | 26 | −4 | 1.53 | |
| L | 2.83 | −30 | 26 | 8 | 1.43 | ||
| R | 2.74 | 38 | 6 | 12 | 5.85 | ||
| Posterior | L | 3.91 | −34 | −2 | 16 | 1.26 | |
| Parahippocampus | |||||||
| Parahippocampal | L | 3.01 | −26 | −42 | −4 | 5.78 | |
| Sub-cortical | |||||||
| Thalamus | R | 2.91 | 10 | −6 | 4 | 3.80 | |
| R | 2.36 | 10 | −14 | 4 | 1.33 | ||
| Brainstem/cerebellum | |||||||
| Cerebellum 7b | L | 3.08 | −30 | −38 | −40 | 4.98 | |
| Cerebellum Crus 1 | R | 2.33 | 42 | −46 | −32 | 1.56 | |
| L | 3.41 | −38 | −50 | −32 | 7.29 | ||
| R | 2.87 | 50 | −62 | −40 | 3.26 | ||
| L | 2.56 | −30 | −82 | −28 | 7.03 | ||
| Cerebellum 8 | L | 2.96 | −38 | −46 | −52 | 1.06 | |
| Cerebellum Crus 2 | L | 2.95 | −46 | −50 | −44 | 2.89 | |
| Cerebellum 6 | L | 2.31 | −6 | −66 | −16 | 5.22 | |
| Pons | 3.41 | −10 | −22 | −24 | 5.13 | ||
| 2.73 | 10 | −22 | −20 | 4.22 | |||
| Negative (Patients V1 < Controls V1) | No statistically significant differences. | ||||||
| Fronto-parietal network (left) | |||||||
| Positive (Patients V1 > Controls V1) | No statistically significant differences | ||||||
| Negative (Patients V1 < Controls V1) | Cortical | ||||||
| Frontal | |||||||
| Superior orbital | R | 2.86 | 26 | 62 | 0 | 1.17 | |
| R | 3.22 | 22 | 34 | 32 | 2.54 | ||
| Middle orbital | R | 3.21 | 50 | 42 | 12 | 3.93 | |
| Superior medial | L | 2.39 | −10 | 62 | 16 | 1.29 | |
| Superior | L | 3.94 | −22 | 10 | 64 | 2.14 | |
| Parietal | |||||||
| Postcentral | R | 3.36 | 58 | −14 | 44 | 1.24 | |
| L | 3.26 | −26 | −30 | 64 | 1.19 | ||
| Supramarginal | R | 2.96 | 66 | −38 | 32 | 1.29 | |
| Superior | R | 3.51 | 30 | −66 | 52 | 2.50 | |
| Occipital | |||||||
| Rolandic_Operculum | R | 2.86 | 62 | 6 | 12 | 3.12 | |
| R | 3.64 | 62 | −18 | 16 | 8.57 | ||
| Middle | L | 2.61 | −46 | −74 | 12 | 1.48 | |
| Temporal | |||||||
| Pole middle | L | 3.28 | −46 | 14 | −28 | 1.07 | |
| Middle | R | 3.83 | 58 | −10 | −20 | 1.83 | |
| Cingulum | |||||||
| Middle | L | 3.46 | −10 | −30 | 44 | 1.70 | |
| Brainstem/cerebellum | |||||||
| Cerebellum 8 | L | 2.86 | −30 | −46 | −52 | 2.42 | |
| Vermis 4 5 | 2.97 | 6 | −54 | −20 | 1.17 | ||
| Vermis 8 | 4.03 | 2 | −66 | −32 | 2.49 | ||
| Cerebellum Crus 2 | L | 2.58 | −38 | −74 | −48 | 1.62 | |
3.3.2.1. Salience network (SN)
The salience network displayed only increased connectivity for CRPS patients compared to controls. Cortically increased connectivity was observed for the frontal superior, medial, inferior triangular, supplemental motor area, orbital, precentral, supramarginal, parietal superior, inferior, precuneus, angular, occipital rolandic operculum, calcarine, superior, inferior, middle, temporal superior middle, inferior, lingual, fusiform, anterior cingulate, and parahippocampus. Subcortically, the basal ganglia (caudate, putamen, pallidum) and hypothalamus showed increased connectivity in CRPS patients compared to controls. The cerebellum displayed increased connectivity across several regions (4, 5, 3, 6, 8, Crus 1).
3.3.2.2. Central executive network (CEN)
The CEN indicated large differences between CRPS patients and controls: cortically frontal superior, middle, orbital, inferior triangular, operculum, rectus, postcentral, precuneus, parietal inferior, occipital superior, inferior, temporal inferior, middle, fusiform, Heschl, anterior cingulate, anterior, posterior insula; subcortically the amygdala and several cerebellar subdivisions (6, 7b, Crus 1) displayed increased connectivity in patients vs. controls. No brain areas displayed decreased connectivity with the CEN in CRPS patients vs. controls.
3.3.2.3. Default mode network (DMN)
For the DMN we only observed increased connectivity in the disease state compared to the control group. The predominant differences for increased connectivity in CRPS patients involved cortical areas (frontal superior, medial, postcentral, supramarginal, precuneus, parietal superior, occipital superior and calcarine and temporal superior).
3.3.2.4. Sensorimotor network (SMN)
Increased connectivity in the CRPS state was observed in several cortical areas (frontal superior medial, middle, orbital, supplemental motor area, postcentral, supramarginal, parietal superior, inferior, occipital middle, cuneus, calcarine, temporal superior, superior pole, middle, inferior, middle anterior cingulate, anterior and posterior insula). Subcortically, differences were observed in the basal ganglia (caudate, putamen), and hippocampus. The cerebellum displayed differences across several regions (4, 5, 6, 8, 9, Crus 1). We also observed decreased connectivity in the CRPS group compared to controls in the frontal superior, middle, inferior triangular, orbital, post-central, precuneus, angular, inferior parietal, anterior/posterior and middle cingulate, temporal middle pole, Heschl, fusiform, lingual, and parahippocampus. Subcortically, decreased connectivity in CRPS patients was observed in the hypothalamus, thalamus and caudate. Cerebellar structures (8, Crus 1, Crus 2) also displayed reduced connectivity.
3.3.2.5. Fronto-parietal network—right (RFPN)
The RFPN displayed only increased connectivity of patients vs. controls. Cortically, increased connectivity was observed in frontal superior, middle, orbital, inferior triangular, operculum, supplemental motor area, paracentral lobule, precentral, supramarginal, postcentral, parietal inferior, occipital middle, calcarine, temporal superior, middle, inferior, temporal pole, anterior, mid-cingulate, anterior, posterior insula; subcortically the thalamus and cerebellar subdivisions (6, 7b, 8, Crus 1, Crus 2).
3.3.2.6. Fronto-parietal network—left (LFPN)
The LVDN displayed only decreased connectivity of patients vs. controls with the following structures: cortically; frontal superior, orbital, postcentral, supramarginal, parietal superior, rolandic operculum, occipital middle, temporal middle, temporal pole, and parahippocampus, no subcortical brain structures and some cerebellar subdivisions (4 5, 8, Crus 2).
3.3.2.7. Summary of observed differences for disease effect
In the disease state, there were significant differences in most networks except in visual associated networks. Several networks displayed only increased connectivity in CRPS patients vs. controls (SN, CEN, DMN), SMN displayed both increases and decreases in connectivity between CRPS patients and controls (SMN), and only one (LFPN) displayed decreased connectivity in patients vs. controls. The largest volume of increased connectivity was observed in the salience network (285.53 cm3) followed by RFPN (252.30 cm3), SMN (224.31 cm3) and CEN (136.31 cm3). The DMN displayed a smaller increase (13.05 cm3). In examining connectivity in the SMN, there were larger increases than decreases in connectivity (224.31 vs. 179.30 cm3).
3.3.3. Residual effect: comparison of brain networks for CRPS visit 2 and Controls visit 2
The following remaining differences in connectivity were observed (Figs. 5 and 6 and Table 4).
Table 4.
Residual effects: The table indicates brain areas of significant increased or decreased connectivity with the listed networks for CRPS Patients visit 2 vs. Controls visit 2. Coordinates and max statistical value (z-stat) are given for peak activity as well as volume (Vol) of each cluster of activity. See Materials and methods for details.
| Brain region | Lat. | z-Stat | X (mm) | Y (mm) | Z (mm) | Vol (cm) | |
|---|---|---|---|---|---|---|---|
| Salience | |||||||
| Positive (Patients visit 2 > Controls visit 2) | No statistically significant differences | ||||||
| Negative (Patients visit 2 < Controls visit 2) | Cortical | ||||||
| Frontal | |||||||
| Precentral | L | 3.46 | −50 | −2 | 32 | 1.00 | |
| Parietal | |||||||
| Postcentral | L | 3.18 | −34 | −38 | 60 | 1.18 | |
| Temporal | |||||||
| Inferior | R | 3.51 | 54 | −50 | −12 | 2.12 | |
| Central executive network (CEN) | |||||||
| Positive (Patients visit 2 > Controls visit 2) | Cortical | ||||||
| Occipital | |||||||
| R | 3.37 | 62 | −34 | −16 | 1.46 | ||
| Negative (Patients visit 2 < Controls visit 2) | Cortical | ||||||
| Parietal | |||||||
| Postcentral | R | 2.53 | 34 | −34 | 48 | 1.20 | |
| Occipital | |||||||
| Calcarine | L | 3.28 | −10 | −50 | 4 | 1.42 | |
| R | 2.63 | 6 | −86 | 12 | 1.25 | ||
| Default mode network | |||||||
| Positive (Patients visit 2 > Controls visit 2) | No statistically significant differences | ||||||
| Negative (Patients visit 2 < Controls visit 2) | Cortical | ||||||
| Frontal | |||||||
| Middle orbital | R | 2.73 | 26 | 50 | 24 | 1.95 | |
| R | 3.07 | 34 | 34 | 36 | 1.06 | ||
| R | 2.35 | 38 | 6 | 36 | 1.14 | ||
| Inferior orbital | R | 3.82 | 30 | 34 | −16 | 2.30 | |
| Middle | L | 3.22 | −22 | 42 | 24 | 2.00 | |
| L | 2.39 | −50 | 26 | 32 | 1.86 | ||
| L | 3.05 | −38 | 2 | 52 | 1.29 | ||
| Precentral | R | 3.53 | 58 | 2 | 24 | 1.57 | |
| L | 3.00 | −46 | 2 | 48 | 1.02 | ||
| L | 3.85 | −58 | −2 | 36 | 1.44 | ||
| L | 2.54 | −34 | −22 | 56 | 1.77 | ||
| Parietal | |||||||
| Supramarginal | L | 3.12 | −62 | −30 | 40 | 2.77 | |
| Postcentral | L | 3.60 | −38 | −38 | 64 | 3.45 | |
| Precuneus | R | 2.35 | 22 | −46 | 12 | 1.19 | |
| Occipital | |||||||
| Superior | R | 3.70 | 26 | −78 | 20 | 8.43 | |
| Middle | L | 3.46 | −22 | −94 | 12 | 1.78 | |
| Temporal | |||||||
| Fusiform | L | 2.79 | −30 | −6 | −40 | 1.78 | |
| Superior | R | 3.58 | 62 | −14 | 0 | 1.26 | |
| Inferior | R | 2.62 | 62 | −38 | −16 | 2.19 | |
| Middle | L | 3.15 | −62 | −54 | 16 | 1.28 | |
| L | 3.35 | −54 | −66 | 8 | 3.34 | ||
| Lingual | R | 2.88 | 18 | −66 | −12 | 1.43 | |
| L | 3.06 | −2 | −66 | 4 | 3.09 | ||
| Parahippocampus | |||||||
| Parahippocampal | L | 3.33 | −18 | −6 | −32 | 1.70 | |
| Sub-cortical | |||||||
| Putamen | R | 3.73 | 30 | 2 | 0 | 2.68 | |
| Brainstem/cerebellum | |||||||
| msn | R | 2.47 | 6 | −34 | −48 | 1.21 | |
| Cerebellum 9 | L | 3.31 | −18 | −42 | −52 | 2.26 | |
| Cerebellum 4 5 | R | 3.93 | 10 | −50 | −8 | 1.66 | |
| Cerebellum Crus 2 | L | 3.51 | −18 | −74 | −36 | 2.08 | |
| L | 3.22 | −6 | −86 | −32 | 1.26 | ||
| Cerebellum Crus 1 | R | 3.23 | 22 | −74 | −36 | 1.15 | |
| L | 3.90 | −26 | −86 | −32 | 2.20 | ||
| Sensorimotor network | |||||||
| Positive (Patients visit 2 > Controls visit 2) | No statistically significant differences | ||||||
| Negative (Patients visit 2 < Controls visit 2) | Cortical | ||||||
| Parietal | |||||||
| Precuneus | L | 3.29 | −6 | −46 | 8 | 1.04 | |
| Cerebellum | |||||||
| Positive (Patients visit 2 > Controls visit 2) | No statistically significant differences | ||||||
| Negative (Patients visit 2 < Controls visit 2) | Cortical | ||||||
| Frontal | |||||||
| Superior medial | R | 3.09 | 22 | 62 | −8 | 1.56 | |
| Middle | L | 3.76 | −30 | 10 | 64 | 1.28 | |
| Superior | R | 3.59 | 2 | 34 | 56 | 1.42 | |
| Supp. motor area | R | 3.43 | 10 | −10 | 68 | 1.31 | |
| Paracentral lobule | L | 2.95 | −10 | −18 | 72 | 1.18 | |
| Occipital | |||||||
| Calcarine | L | 3.18 | −2 | −98 | 4 | 1.64 | |
| Superior | L | 2.73 | −18 | −70 | 28 | 1.34 | |
| Temporal | |||||||
| Pole middle | R | 3.43 | 38 | 18 | −40 | 1.16 | |
| Middle | R | 2.61 | 62 | −50 | 16 | 2.20 | |
| Parahippocampus | |||||||
| Parahippocampal | R | 3.59 | 22 | −2 | −28 | 3.18 | |
| Sub-cortical | |||||||
| Caudate | L | 2.85 | −14 | −2 | 20 | 1.01 | |
| Brainstem / cerebellum | |||||||
| Cerebellum_Crus 1 | L | 4.06 | −38 | −78 | −20 | 3.46 | |
| Fronto-parietal network (right) | |||||||
| Positive (Patients visit 2 > Controls visit 2) | Cortical | ||||||
| Frontal | |||||||
| Frontal pole | R | 2.95 | 2 | 58 | 0 | 3.46 | |
| Superior medial | L | 2.51 | −10 | 50 | 32 | 2.02 | |
| Superior orbital | R | 3.03 | 14 | 2 | 72 | 1.27 | |
| Middle orbital | R | 2.58 | 26 | 38 | 32 | 1.75 | |
| R | 3.50 | 34 | 30 | 40 | 4.18 | ||
| Inferior orbital | R | 3.19 | 38 | 38 | −8 | 2.36 | |
| Middle | L | 3.27 | −38 | 30 | 44 | 1.69 | |
| Supp. motor area | L | 2.53 | −10 | 6 | 72 | 1.02 | |
| L | 2.72 | −10 | −14 | 52 | 1.51 | ||
| Paracentral lobule | L | 3.68 | −10 | −30 | 64 | 2.26 | |
| Parietal | |||||||
| Postcentral | L | 2.66 | −42 | −30 | 48 | 1.12 | |
| Inferior | R | 3.32 | 46 | −38 | 56 | 2.22 | |
| R | 2.56 | 38 | −38 | 48 | 1.66 | ||
| Precuneus | R | 2.62 | 6 | −66 | 60 | 1.06 | |
| Occipital | |||||||
| Calcarine | R | 2.49 | 22 | −90 | 0 | 1.38 | |
| Middle | L | 2.28 | −18 | −94 | 4 | 1.30 | |
| Temporal | |||||||
| Inferior | L | 4.51 | −42 | 2 | −36 | 6.86 | |
| L | 3.10 | −46 | −46 | −12 | 1.62 | ||
| Middle | L | 2.26 | −62 | −18 | −24 | 1.22 | |
| R | 3.44 | 62 | −54 | 12 | 2.66 | ||
| Fusiform | R | 3.70 | 26 | −78 | −4 | 2.69 | |
| Superior | R | 2.43 | 54 | −46 | 20 | 2.50 | |
| Cingulum | |||||||
| Middle | L | 2.60 | −2 | 18 | 32 | 1.02 | |
| Insula | |||||||
| Posterior | L | 2.52 | −34 | −26 | 20 | 1.19 | |
| Parahippocampus | |||||||
| Parahippocampal | L | 3.20 | −22 | −10 | −36 | 2.04 | |
| Sub-cortical | |||||||
| Putamen | R | 3.20 | 30 | 6 | 8 | 3.29 | |
| Hippocampus | R | 3.05 | 38 | −26 | −8 | 1.09 | |
| Brainstem/cerebellum | |||||||
| Cerebellum 8 | L | 2.48 | −34 | −46 | −48 | 1.15 | |
| Cerebellum Crus 1 | R | 2.57 | 46 | −54 | −32 | 3.24 | |
| L | 2.68 | −26 | −74 | −36 | 1.63 | ||
| Cerebellum 6 | R | 2.99 | 10 | −70 | −24 | 3.68 | |
| Pons | L | 3.08 | −2 | −18 | −36 | 3.44 | |
| Brainstem | 2.52 | 2 | −34 | −32 | 2.17 | ||
| Negative (Patients visit 2 < Controls visit 2) | Cortical | ||||||
| Frontal | |||||||
| Middle orbital | R | 3.19 | 42 | 14 | 52 | 2.16 | |
| R | 3.06 | 30 | −2 | 56 | 1.17 | ||
| Parietal | |||||||
| Precuneus | L | 3.01 | −10 | −50 | 24 | 1.21 | |
| Occipital | |||||||
| Rolandic operculum | L | 1.73 | −54 | −6 | 8 | 1.25 | |
| Temporal | |||||||
| Superior | L | 2.07 | −54 | −2 | −4 | 1.10 | |
| Inferior | R | 3.00 | 50 | −6 | −32 | 1.43 | |
| Lingual | R | 2.10 | 22 | −90 | −12 | 1.39 | |
| Sub-cortical | |||||||
| Putamen | L | 2.46 | −14 | 10 | 0 | 2.17 | |
| Hippocampus | R | 2.58 | 34 | −14 | −24 | 1.03 | |
| Brainstem/cerebellum | |||||||
| Cerebellum 8 | R | 2.89 | 34 | −42 | −44 | 1.66 | |
| Fronto-parietal network (left) | |||||||
| Positive (Patients visit 2 > Controls visit 2) | No statistically significant differences | ||||||
| Negative (Patients visit 2 < Controls visit 2) | Cortical | ||||||
| Frontal | |||||||
| Superior | L | 3.56 | −22 | 10 | 64 | 1.69 | |
| Brainstem/cerebellum | |||||||
| Vermis 3 | 4.55 | 2 | −46 | −16 | 1.63 | ||
3.3.3.1. SN
Very small differences in connectivity were observed for this network with decreased connectivity in the precentral/postcentral and inferior temporal cortices in CRPS patients.
3.3.3.2. CEN
For this network, also few differences in connectivity were observed with increased connectivity in precuneus, inferior temporal and cerebellum Crus 1 decreased connectivity in postcentral area and calcarine.
3.3.3.3. DMN
The DMN displayed only decreased connectivity in CRPS patients vs. controls; cortically in orbital, middle, superior frontal, precentral, postcentral, supramarginal, precuneus, superior, middle occipital, fusiform, temporal superior, middle, inferior, lingual, anterior mid-cingulate and parahippocampus; subcortically in putamen, and cerebellar structures (4 5, 9, Crus 1, Crus 2).
3.3.3.4. SMN
The SMN displayed small differences and only decreased connectivity (precuneus, fusiform).
3.3.3.5. RFPN
Several structures displayed increased connectivity of patients vs. controls. Cortically; mid-frontal, orbital, supplemental motor area, postcentral, nferior parietal, precuneus, mid-occipital, calcarine, superior, middle, inferior temporal, fusiform, mid-anterior cingulate, posterior insula, and parahippocampus; subcortically putamen and hippocampus and cerebellar subdivisions (6,8, Crus 1). Decreased connectivity was observed in middle frontal, orbital, supramarginal, precuneus, rolandic operculum, temporal superior, inferior, fusiform, lingual; subcortically putamen and hippocampus and cerebellum 8.
3.3.3.6. LFPN
Only small reductions in connectivity of patients vs. controls (superior frontal and vermis 3) were observed.
3.3.4. Summary of residual effects
The SN displayed very small differences between patients and controls (4.30 cm3 decreased connectivity). The CEN also displayed small differences (5.50 cm3 increased and 3.87 cm3 decreased connectivities). The DMN had significant reduced connectivity (70.36 cm3). The SMN had small differences in connectivity (2.01 cm3 decreased connectivity). RFPN had significant differences (66.52 cm3 increased connectivity, 18.51 cm3 decreased connectivity). LFPN had small decrease in connectivity (3.32 cm3).
3.4. Treatment effects: patient's correlation with VAS (spontaneous pain) pre/post-treatment
We observed correlation of connectivity strength with spontaneous pain ratings (Figs. 5 and 6 and Table 5) in the following networks.
Table 5.
Treatment effects: Correlation with spontaneous pain scores: The table indicates brain areas of significant increased or decreased connectivity according to spontaneous pain scores in patients pre- and post-treatment. Brain areas of decreased connectivity with decreased pain scores appear in red–yellow, areas of increased connectivity with decreased pain scores appear in blue–light blue. Coordinates and max statistical value (z-stat) are given for peak activity as well as volume (Vol) of each cluster of activity. See Materials and methods for details.
| Brain region | Lat. | z-stat | X (mm) | Y (mm) | Z (mm) | Vol (cm) | |
|---|---|---|---|---|---|---|---|
| Salience | |||||||
| Positive (patients V1 > controls V1). | Cortical | ||||||
| Frontal | |||||||
| Middle_Orbital | R | 4.87 | 34 | 38 | 24 | 1.15 | |
| Middle_Orbital | R | 4.26 | 30 | 26 | 40 | 1.10 | |
| Inferior_Orbital | R | 7.08 | 50 | 22 | −4 | 1.50 | |
| Negative (increased connectivity with decreased pain) | Cortical | ||||||
| Frontal | |||||||
| Middle | R | 5.45 | 6 | 58 | −4 | 1.03 | |
| Superior_Orbital | R | 4.97 | 26 | 62 | 16 | 2.78 | |
| Rectus | L | 4.32 | −2 | 22 | −20 | 1.14 | |
| Inferior_Operculum | R | 4.32 | 50 | 6 | 20 | 3.44 | |
| Occipital | |||||||
| Rolandic_Operculum | L | 4.34 | −46 | 2 | 12 | 1.50 | |
| Temporal | |||||||
| Superior | R | 3.98 | 46 | −2 | −12 | 2.15 | |
| Superior | R | 3.51 | 38 | −30 | 12 | 1.17 | |
| Subcortical | |||||||
| Hippocampus | L | 5.00 | −14 | −10 | −20 | 1.20 | |
| Central executive | |||||||
| Positive (decreased connectivity with decreased pain) | Cortical | ||||||
| Temporal | |||||||
| Parahippocampus | R | 4.90 | 22 | 10 | −24 | 3.84 | |
| Negative (increased connectivity with decreased pain) | Cortical | ||||||
| Frontal | |||||||
| Middle | R | 4.87 | 10 | 46 | −12 | 1.42 | |
| Inferior_Orbital | L | 4.92 | −42 | 42 | −16 | 1.20 | |
| Inferior_Triangular | R | 3.38 | 54 | 26 | 0 | 2.02 | |
| Inferior_Orbital | L | 6.84 | −26 | 26 | −12 | 4.72 | |
| Cortical | |||||||
| Frontal | |||||||
| Parietal | |||||||
| Postcentral | L | 5.05 | −46 | −10 | 28 | 1.23 | |
| Postcentral | L | 4.00 | −54 | −18 | 36 | 1.96 | |
| Occipital | |||||||
| Middle | L | 3.51 | −42 | −74 | 16 | 1.04 | |
| Superior | L | 6.64 | −18 | −86 | 8 | 1.56 | |
| Temporal | |||||||
| Middle | L | 3.69 | −58 | −18 | 0 | 1.28 | |
| Middle | L | 3.91 | −58 | −34 | 4 | 1.22 | |
| Middle | L | 3.64 | −54 | −34 | −8 | 1.70 | |
| Middle | R | 5.73 | 42 | −58 | 12 | 1.18 | |
| Middle | R | 3.83 | 42 | −70 | 8 | 1.50 | |
| Inferior | L | 3.46 | −50 | −22 | −20 | 1.70 | |
| Fusiform | L | 6.94 | −30 | −50 | −8 | 2.92 | |
| Parahippocampal | R | 3.83 | 38 | −30 | −16 | 3.86 | |
| Cingulate | |||||||
| Middle | L | 3.97 | −10 | 2 | 44 | 1.09 | |
| Middle | R | 10.77 | 6 | −38 | 32 | 1.86 | |
| Middle | L | 8.11 | −6 | −38 | 44 | 1.22 | |
| Subcortical | |||||||
| Putamen | R | 4.06 | 18 | 14 | 0 | 1.08 | |
| Brainstem/cerebellum | |||||||
| Cerebellum_Crus 2 | R | 3.63 | 22 | −78 | −40 | 1.16 | |
3.4.1.1. DMN
The DMN displayed positive correlation with VAS indicating decreased connectivity with decreased pain scores in orbital, precentral, superior medial frontal, cuneus, fusiform, temporal inferior, middle, anterior insula, and cerebellum 8. No anti-correlated brain structures with VAS were statistically significant. Aggregate volume for positive correlation was 22.09 cm3.
3.4.1.2. SN
Brain structures that displayed positive connectivity correlation with VAS scores were orbital and middle frontal cortices (i.e., there was reduction in connectivity of SN with brain structures when pain was reduced). Areas that indicated a negative correlation (i.e., increase connectivity when pain scores decreased) included cortically: orbital, rectus, inferior operculum, rectus middle frontal, temporal superior, rolandic operculum, and subcortically hippocampus. Total volumes of significant correlation were 4.72 cm3. Volumes of anti-correlated structures were 15.35 cm3.
3.4.1.3. CEN
The CEN only displayed the parahippocampus to be positively correlated with VAS scores (3.84 cm3). Several brain areas were found to be anti-correlated with VAS and include frontal middle, orbital, inferior triangular, postcentral, angular, inferior and middle temporal gyri, fusiform, mid-cingulate, parahippocampus, subcortically; putamen and cerebellum (8 and Crus 2). Anticorrelated areas amounted to 40.52 cm3.
3.4.1.4. SMN
The SMN had correlated connectivity changes with pain scores in several cortical areas frontal (orbital, rectus, operculum), superior parietal and parahippocampal areas. Anti-correlated areas appear in cortical (orbital, superior and inferior temporal, middle occipital). The SMN displayed similar changes in total volumes of significant correlations; positive correlations resulted in 8.72 cm3. Anti-correlated had 8.76 cm3.
3.4.1.5. Cer
The cerebellum network only indicated anti-correlated changes in connectivity with superior triangular and inferior temporal cortices (2.07).
3.4.1.6. RFPN
Brain areas of positively correlated changes in connectivity with VAS were superior frontal, supplemental motor area, fusiform, temporal superior; subcortically amygdala and putamen and cerebellar areas (7b and 8). Anticorrelated connectivity changes with VAS were observed in inferior orbital, supramarginal, superior and inferior parietal, angular, temporal pole, middle, lingual and cerebellar areas (Crus 1 and vermis 4 5). Positively correlated volume was 23.52 cm3 and anticorrelated was 17.00 cm3.
3.4.1.7. LFPN
No significant changes in connectivity were observed pre- and post-treatment.
3.5. Test–retest comparison (Controls visit 1 vs. Controls visit 2)
We found no statistically significant differences in brain areas for the above networks between visit 1 and visit 2 for controls.
4. Discussion
One of the major features that differentiate pediatric CRPS from adult CRPS is that most pediatric patients recover within a year (Low et al., 2007) suggesting a nervous system that is more resilient. However, even within this population, some pediatric patients remain resistant. Short intensive treatment programs have reportedly been shown to provide significant benefit at discharge and, importantly, observed to last (Logan et al., 2012) suggesting that many of the complex features of CRPS that include brain alterations can be modified. Thus, these patients provide a unique model for studying the condition itself and the neuroplastic changes associated with disease recovery.
Here we report on early changes in brain networks of pediatric CRPS patients who underwent a 3-week intensive treatment program that resulted in improvement in psychophysical and psychological measures. Intrinsic brain network alterations were measured prior to and following treatment: Several intrinsic brain networks displayed significant alterations (fronto-parietal, salience, central executive, default mode, and sensorimotor networks) before treatment. Following treatment, we observed significant reduction of brain network alterations across several networks but not all of them. In parallel with these brain measures, we observed significant improvements as indicated in a number of psychophysical measures, including spontaneous and evoked pain levels, functional disability, motor function, and small but significant improvement in depressive and anxiety symptoms.
4.1. Disease state, treatment, and residual effects
The data on changes in RSNs in CRPS is among the first to be noted in a pediatric population. Below we discuss RSN changes in disease state, residual effect and treatment effect observed in the results. As noted in the above section, the changes in the brain networks represent an altered reorganization of network in the disease state and a reorganization of RSNs towards a normal state with the treatment. Ideally, in the fully reversed disease condition, the networks will be normalized after treatment and the residual effect will indicate no significant differences between controls and treated patients.
4.1.1. Disease state
RSNs have been reported in adult conditions of chronic pain (Balenzuela et al., 2010; Cauda et al., 2010; Tagliazucchi et al., 2010; Kim et al., 2013; Kornelsen et al., 2013; Kilpatrick et al., 2014) and CRPS (Bolwerk et al., 2013), but not for children. Networks that displayed the largest difference in the disease state were targeted for further exploration along with other contrasts of interest.
4.1.1.1. Fronto-parietal networks
The fronto-parietal networks are lateralized networks that involve lateral prefrontal and posterior parietal cortices (Smith et al., 2009; Cole et al., 2013). We observed significant increased connectivity in the right FPN, a network known to reflect self-awareness, perception, pain and somesthesis (Smith et al., 2009). This is consistent with a heightened state of awareness due to their condition. Interestingly, the left PFN indicates a significant decrease in connectivity in the disease state. The network is associated with cognition, language, and memory (Smith et al., 2009). Other pain conditions, such as fibromyalgia (Seo et al., 2012) and migraine (Russo et al., 2012), have reported the decreased connectivity of this network. The persistence of chronic pain in these patients might result in diminished cognitive ability related to working memory, language, and executive function as seen in adults (Smith et al., 2009).
4.1.1.2. Salience network
The salience network (SN) (Seeley et al., 2007) includes the insula and anterior cingulate cortices and is involved in connecting relevant brain regions for the processing of physiologic information (autonomic, sensory information) that is interpreted in the context of relevance and interoception. Patients with chronic pain experience sustained salience that is responsive to both external stimuli (e.g., sensory, visual) and internal brain (connectivity) states (Borsook et al., 2013). In Fig. 5 and Table 3, the anterior insula presents increased connectivity with the SN and CEN networks, given that these networks form part of a nucleus of networks that monitor and determine the inner and outer state of the individual (DMN), while the SN assigns the importance of the condition and the CEN might execute accordingly (Sridharan et al., 2008; Menon and Uddin, 2010), it is possible that the anterior insula provides the pathway for these networks (SN and CEN) to interact. The significant changes in the disease state in the SN are consistent with the major processes going on in these patients that include but not limited to altered sensation (viz., posterior insula involvement), altered autonomic function (viz., anterior insula processing), alterations in encoding rewarding and aversive stimuli (viz., anterior cingulate) through monitoring of emotional salience and cognitive modulation.
4.1.1.3. Default mode network
Prominent differences were observed in the default mode network (DMN), perhaps the best characterized network of all RSNs (Fox et al., 2005). This brain network that characterizes the activity at rest incorporates a number of brain regions including the precuneus, the medial prefrontal cortex, the posterior cingulate and parts of the medial temporal lobe. Here we report increased connectivity in the default mode network in the disease state. We interpret these findings as they may relate to diminish ability to ‘day-dream’ or switch off because of the cognitive load of the ongoing pain process (Seifert and Maihöfner, 2009).
4.1.1.4. Sensorimotor network
Significant differences were observed in the sensorimotor network (SMN), a network that includes the primary somatosensory cortex and motor systems including the supplementary motor region (Smith et al., 2012). Limitations in motor function due to pain have been shown (Sterling et al., 2001; Huge et al., 2011) and were present in all of our CRPS patients. Indeed, a large difference in connectivity was observed in patients compared to controls indicative of an abnormality in the SMN that was normalized following treatment (Figs. 5 and 6). Alterations in sensation are perhaps the most notable subjective and objective (sensory testing) change in these patients (Sethna et al., 2007; Gierthmuhlen et al., 2012). This is consistent with previous findings where abnormal motor cortex function has been reported in CRPS (Maihöfner et al., 2007; Kirveskari et al., 2010), abnormal pain networks interact with central sensorimotor and autonomic pathways (Cohen et al., 2012), and even thinking about moving may increase pain (Moseley et al., 2008).
4.1.1.5. Central executive network
The central executive network (CEN) is involved in high-level cognitive functions including attention and working memory (Bressler and Menon, 2010; Smith et al., 2012). In adults, as many as 65% of CRPS patients have altered neuropsychological changes that have been described as a “dysexecutive syndrome” and some patients present with global cognitive impairment (Libon et al., 2010). Although we did not measure overt alterations in cognitive function among patients in this sample, it is not surprising that this was a prominent network of activity in the disease state given the growing empirical evidence of altered cognitive function among chronic pain patients (Campbell et al., 2005; Deere et al., 2012).
4.1.2. Residual effects
With treatment we observed small differences in all networks (FPN, SN, SMN, CN) noted above when comparing CRPS visit 2 to Controls visit 2 (Fig. 5 and Table 4), suggesting residual effects. Interestingly, the left FPN had small significant differences, perhaps an indication of the patients' recovery of his/her cognitive abilities. The right FPN, however, remained highly interconnected suggesting that the patient, with reduced pain and increased mobility, might still remain vigilant of the affected limb. In any treatment there will be individuals within the group that may not be fully responsive or the treatment effects while providing symptomatic relief do not fully allow for complete reversal from the disease state to a new healthy state. We have observed this in our previous study of pediatric CRPS patients (Lebel et al., 2008) where pain levels were essentially reversed but brain responses to experimental pain remained abnormal at least as defined in the early phase of symptomatic recovery. Specifically, in the latter study, brain regions such basal ganglia and sensorimotor cortex were considered to still be altered in the recovered state (Lebel et al., 2008) in comparing experimental pain applied to the affected and the unaffected limb. Since it is impossible to control for temporal nature of recovery, some networks may remain altered (residual) due to the likelihood that patients are in early symptom recovery vs. full remission or resolution of symptoms. However, based on the treatment paradigm for CRPS patients undergoing the same psychophysical treatment, the beneficial effects (functional gains) persist (Logan et al., 2012). Another option for our observation of residual effect finding may relate to some patients who were more resilient to treatment and thus not complete responders. Thus, residual effects may relate to the severity of the effects of the disease on brain networks.
4.1.3. Treatment effect
The use of RSNs in evaluating treatment effects in clinical conditions has been reported for some conditions in adults (e.g., depression (Abbott et al., 2013; Salomons et al., 2014)) but only a few reports on treatment effects have been reported for chronic pain (Becerra et al., 2009; Hashmi et al., 2014) but we are not aware of any reports in children with chronic pain. We observed significant correlation of network connectivity and spontaneous pain scores (Figs. 5 and 6, Table 5).
4.1.3.1. Fronto-parietal networks
The right FPN displayed several areas that had positively correlated change in connectivity strength with spontaneous pain scores as well as some areas that displayed the opposite correlation. Some of the areas showing positive correlation are associated with fear processing (amygdala, prefrontal areas) suggesting that the reduction could be due to a diminished fear of pain. Some areas, however, increased their connectivity and include structures associated with attention. The left FPN did not show significant changes in connectivity with VAS, although it was significantly different (decreased) in the disease condition and only marginally when compared with controls post-treatment. The relatively small number of patients might be the cause for this lack of significance.
4.1.3.2. Salience and central executive networks
Surprisingly, the SN and CEN networks both display much larger increased connectivity with decrease pain. These networks act in conjunction with the DMN, the latter will maintain vigilance of self, the SN will determine the salience of all the different events monitored by the DMN and finally, the CEN might plan and execute (Menon and Uddin, 2010). We postulate that the significant increase in SN and CEN might be related to a state in which the patient is more capable of performing normal activities. Nevertheless, we found that the SN and CEN networks are similar to controls post-treatment, suggesting that there may be a hierarchy of RSN response with treatment. While a number of brain regions are involved (see Results section), one of the structures involved in the CEN response was the putamen. It is a structure that is activated in pain and analgesia (reviewed in Borsook et al., 2010) and thus its involvement here is consistent with prior studies.
4.1.3.3. Default mode network
Following treatment, the DMN shows a significant positive correlation and we interpret this observation as a concomitant reduction of hyperconnectivity in this network with pain. As the pain decreases, the emotional and vigilant function of the DMN diminishes. This is consistent with the observed reduction of hyperconnectivity of patients post-treatment vs. controls. The changes following treatment suggest a restoration of the DMN that is consistent with the idea that the abnormal balance between positive and negative inputs to the system is restored (see Baliki et al., 2008).
4.1.3.4. Sensorimotor network
The SMN shows a combination of increased and decreased connectivities, likely the combination of movement avoidance as a result of pain related fear in CRPS (de Jong et al., 2005; de Jong et al., 2011; Simons et al., 2014) as well as regaining the ability to control the affected limb (McCabe et al., 2008). The latter process, we suggest, might induce a combination of brain changes that some will be reduced with reduced pain while other connectivities will improve with improved mobility.
4.2. Resting state networks as a model for evaluating changes in brain state in CRPS
Nerve damage associated with CRPS produces alterations in resting state networks that differ from healthy controls. The notion of the utility of RSN (reviewed in van den Heuvel and Hulshoff Pol, 2010) as consistent patterns across studies (Shehzad et al., 2009), measures of brain stability or reproducibility over time (Meindl et al., 2010; Zuo et al., 2010; Patriat et al., 2013), disease state (Greicius et al., 2007; Gottlich et al., 2013) or drug effects (Scheidegger et al., 2012), and other changes in brain state (e.g., cognitive) (Moussa et al., 2011) have been reported across numerous brain conditions. In disease states, the interconnections between brain systems may affect the dominance or organization of RSNs (Poston and Eidelberg, 2012). However, the notion of treatment effects has been less evaluated in part because of the lack of significant improvements in many CNS diseases. The pediatric CRPS model provides a good opportunity to evaluate such changes. One of the ongoing themes in understanding chronic brain disease relates to its resilience to treatments. Understanding how effective treatments may ‘unwind’ the disease state can provide insights to the neurobiology of the disease and targets for treatments (i.e., brain state dependent targets such as the sensorimotor or salience networks). Furthermore, chronic pain conditions that begin in childhood may have long lasting effects into adulthood (Campo et al., 2001; Tan et al., 2009; Walker et al., 2010; Shelby et al., 2013). In our prior study of CRPS for example, evoked fMRI measures showed a persistence of abnormal brain systems albeit that the subjects' pain had diminished or disappeared (Lebel et al., 2008). This supports the issue that subjective changes and objective changes may be coursing in the same direction, but not with the same temporal characteristics. It should be noted that our RSN analysis for healthy subjects was consistent with the literature for children (Fair et al., 2008) and showed significant overlap for RSNs reported in the adult literature (see Fig. 4). As such, the basic comparisons in this study would seem to provide a robust basis for evaluation of altered RSN in the disease state, albeit in a pediatric population where there is still ongoing brain development (Hoff et al., 2013).
4.3. CRPS: pediatric vs. adult brain alterations
Adult patients with CRPS have predominantly reduced connectivity of the DMN compared to controls (Bolwerk et al., 2013), in contrast to other chronic pain conditions such as fibromyalgia or lower back pain that tend to have a hyper-connected DMN (Ichesco et al., 2014; Li et al., 2014). In pediatric CRPS patients, we found mostly increased connectivity of the DMN in patients compared to controls. This is the most significant difference between children and adults. All of the other networks with alterations, described below, otherwise seem to agree between children and adults. The DMN, CEN and SN are networks that dynamically alter each other (Sridharan et al., 2008; Goulden et al., 2014). A possible explanation for the adult condition is the excessive attention to the pain which maintains the CEN to be highly active and hence the DMN becomes mostly inhibited. In children, the CEN is not as developed as in adults and potentially their behavior towards pain is more of an emotional nature maintaining engagement of the DMN and SN predominantly; indeed functional coupling in adults is stronger in healthy adults compared with children (Uddin et al., 2011), which may provide a basis for the observed differences.
The fronto-parietal network in adults has increased connectivity in patients compared to controls. A related network that we detected in the pediatric patients, the right fronto-parietal network, associated with pain perception/somesthesis (Smith et al., 2009), also indicated increased connectivity changes in patients vs. controls. However, the left fronto-parietal network, associated with cognition/memory (Smith et al., 2009), has reduced connectivity in the disease state in pediatric patients and would indicate neuropsychological impairment as reported in the literature (Cruz et al., 2011). Although no functional imaging studies of adults have been reported, a neuropsychological study (Libon et al., 2010) also found cognitive deficits in memory/verbal fluency in CRPS patients. Another potential difference may relate to differences in the ability of children with respect to voluntary behavior that differs because of their stage of development (Alahyane et al., 2014). Potential explanations for the observed difference between adults and children are unclear but may relate to (1) specific treatments; (2) relative state of brain development and particularly the frontal areas and their connections (Selemon, 2013); or (3) relative short duration of the disease in children (see Low et al., 2007). However, even in adults, the definition of CRPS sub-groups (i.e., phenotypes) makes evaluations complex (Cossins et al., 2013).
4.4. Caveats
There are several issues that might affect the generalizability of our results and they are discussed below.
4.4.1. Cohort size
The number of subjects per group is relatively low. This is in part a reflection of the difficulty of performing pediatric studies and especially in children with chronic pain issues. Nevertheless, the results suggest robust results that mirror what has been observed in adult CRPS and that such changes tend to revert with treatment.
4.4.2. Order effects
It is virtually impossible to recruit CRPS subjects who are without pain and then with pain in order to randomize the scanning order in our CRPS patients. However, (a) our control subjects also participated in two study sessions, therefore the order effect should be minimized in the comparisons of CRPS subjects to controls; (b) no differences in brain networks were observed between scan 1 and scan 2 for the control group; and (c) patients remained on the same medications for both scanning sessions, thus controlling for pharmacological effects.
4.4.3. Age/menses effects
Post-pubertal hormonal changes might be related to increased prevalence of chronic pain in young women where 71% of CRPS patients are post-pubertal girls (Kachko et al., 2008). The majority of the girls in our study were post-pubertal (7 out of 9 see Table 1) and we cannot exclude the possibility that some of the observed changes are related to hormonal/menstrual changes in our participants, although it would be hypothesized that the impact of hormones would actually increase pain and thus attenuate our observed findings.
4.4.4. CRPS subtypes
In adults, distinct CRPS subgroups have been defined, suggesting three possible CRPS groups: (1) a relatively limited syndrome with vasomotor signs predominating; (2) a relatively limited syndrome with neuropathic pain/sensory abnormalities predominating; and (3) a florid CRPS syndrome similar to “classic RSD” descriptions (Bruehl et al., 2002). While some variability of symptoms existed in our CRPS sample, the small number of subjects precluded us from analyzing CRPS subtypes.
4.4.5. Drug effects
The fact that the patient cohort is typically on medication (Table 1) poses an inherent limitation in conducting a study such as the one described here. We asked our CRPS patients to refrain from using medication for 4 h prior to scanning, in order to minimize the acute effects of the drug on functional brain activity.
4.4.6. Sex differences
As with many pain disorders, there is a predominance of females in the cohort evaluated. Future studies that manage to recruit larger numbers of males would allow for sex-related differences to be evaluated.
5. Conclusions
Measures of RSN are useful for longitudinal studies of clinical conditions (Fox and Raichle, 2007) and that these correlate with structural connectivity in the brain (van den Heuvel et al., 2009). Here we report how these measures may be used to evaluate the clinical condition of pediatric CRPS. Given the increased neuronal plasticity during development, it is possible that therapies that are effective in reducing pain symptoms in children will also reverse the physiologic abnormalities observed in the brain. In the current study we observed robust CRPS-related brain network alterations in networks associated with pain processing (salience, default mode, central executive, sensorimotor networks). Furthermore several of the observed changes revert following treatment.
Acknowledgments
This work was supported by a grant from NINDS R01NS065051 (DB), Radiology and Anesthesia Foundations, Boston Children's Hospital (DB and LB), NINDS K24 NS064050 (DB), NICHD K23 HD067202 (LS), and Sara Page Mayo Endowment for Pediatric Pain Research and Treatment (CB).
References
- Abbott C.C., Lemke N.T., Gopal S., Thoma R.J., Bustillo J., Calhoun V.D., Turner J.A. Electroconvulsive therapy response in major depressive disorder: a pilot functional network connectivity resting state FMRI investigation. Frontiers in Psychiatry. 2013;4:10. doi: 10.3389/fpsyt.2013.00010. 23459749 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alahyane N., Brien D.C., Coe B.C., Stroman P.W., Munoz D.P. Developmental improvements in voluntary control of behavior: effect of preparation in the fronto-parietal network? NeuroImage. 2014;98:103–117. doi: 10.1016/j.neuroimage.2014.03.008. 24642280 [DOI] [PubMed] [Google Scholar]
- Azari P., Lindsay D.R., Briones D., Clarke C., Buchheit T., Pyati S. Efficacy and safety of ketamine in patients with complex regional pain syndrome: a systematic review. CNS Drugs. 2012;26:215–228. doi: 10.2165/11595200-000000000-00000. 22136149 [DOI] [PubMed] [Google Scholar]
- Balenzuela P., Chernomoretz A., Fraiman D., Cifre I., Sitges C., Montoya P., Chialvo D.R. Modular organization of brain resting state networks in chronic back pain patients. Frontiers in Neuroinformatics. 2010;4:116. doi: 10.3389/fninf.2010.00116. 21206760 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baliki M.N., Geha P.Y., Apkarian A.V., Chialvo D.R. Beyond feeling: chronic pain hurts the brain, disrupting the default-mode network dynamics. Journal of Neuroscience: the Official Journal of the Society for Neuroscience. 2008;28:1398–1403. doi: 10.1523/JNEUROSCI.4123-07.2008. 18256259 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Becerra L., Schwartzman R.J., Kiefer R.T., Rohr P., Moulton E.A., Wallin D., Pendse G., Morris S., Borsook D. CNS measures of pain responses pre- and post-anesthetic ketamine in a patient with complex regional pain syndrome. Pain Medicine (Malden, Mass.) 2009 doi: 10.1111/pme.12939. 19254342 [DOI] [PubMed] [Google Scholar]
- Beckmann C.F., DeLuca M., Devlin J.T., Smith S.M. Investigations into resting-state connectivity using independent component analysis. Philosophical Transactions of the Royal Society of London. Series B, Biological Sciences. 2005;360:1001–1013. doi: 10.1098/rstb.2005.1634. 16087444 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bijsterbosch J., Smith S., Forster S., John O.P., Bishop S.J. Resting state correlates of subdimensions of anxious affect. Journal of Cognitive Neuroscience. 2014;26:914–926. doi: 10.1162/jocn_a_00512. 24168223 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Binkley J.M., Stratford P.W., Lott S.A., Riddle D.L. The Lower Extremity Functional Scale (LEFS): scale development, measurement properties, and clinical application. North American Orthopaedic Rehabilitation Research Network. Physical Therapy. 1999;79:371–383. 10201543 [PubMed] [Google Scholar]
- Bohr I.J., Kenny E., Blamire A., O'Brien J.T., Thomas A.J., Richardson J., Kaiser M. Resting-state functional connectivity in late-life depression: higher global connectivity and more long distance connections. Frontiers in Psychiatry. 2012;3:116. doi: 10.3389/fpsyt.2012.00116. 23316175 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bolwerk A., Seifert F., Maihöfner C. Altered resting-state functional connectivity in complex regional pain syndromeJournal of Pain: Official Journal of the American Pain Society. 2013;14:1107–1115. doi: 10.1016/j.jpain.2013.04.007. 23791136 [DOI] [PubMed] [Google Scholar]
- Borsook D., Upadhyay J., Chudler E.H., Becerra L. A key role of the basal ganglia in pain and analgesia — insights gained through human functional imaging. Molecular Pain. 2010;6:27. doi: 10.1186/1744-8069-6-27. 20465845 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borsook D., Edwards R., Elman I., Becerra L., Levine J. Pain and analgesia: the value of salience circuits. Progress in Neurobiology. 2013;104:93–105. doi: 10.1016/j.pneurobio.2013.02.003. 23499729 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bressler S.L., Menon V. Large-scale brain networks in cognition: emerging methods and principles. Trends in Cognitive Sciences. 2010;14:277–290. doi: 10.1016/j.tics.2010.04.004. 20493761 [DOI] [PubMed] [Google Scholar]
- Bruehl S., Harden R.N., Galer B.S., Saltz S., Backonja M., Stanton-Hicks M. Complex regional pain syndrome: are there distinct subtypes and sequential stages of the syndrome? Pain. 2002;95:119–124. doi: 10.1016/s0304-3959(01)00387-6. 11790474 [DOI] [PubMed] [Google Scholar]
- Bruehl S., Harden R.N., Galer B.S., Saltz S., Bertram M., Backonja M., Gayles R., Rudin N., Bhugra M.K., Stanton-Hicks M. External validation of IASP diagnostic criteria for complex regional pain syndrome and proposed research diagnostic criteria. International Association for the Study of Pain. Pain. 1999;81:147–154. doi: 10.1016/s0304-3959(99)00011-1. 10353502 [DOI] [PubMed] [Google Scholar]
- Campbell C.M., Edwards R.R., Fillingim R.B. Ethnic differences in responses to multiple experimental pain stimuli. Pain. 2005;113:20–26. doi: 10.1016/j.pain.2004.08.013. 15621360 [DOI] [PubMed] [Google Scholar]
- Campo J.V., Di Lorenzo C., Chiappetta L., Bridge J., Colborn D.K., Gartner J.C., Jr., Gaffney P., Kocoshis S., Brent D. Adult outcomes of pediatric recurrent abdominal pain: do they just grow out of it? Pediatrics. 2001;108:E1. doi: 10.1542/peds.108.1.e1. 11433080 [DOI] [PubMed] [Google Scholar]
- Cauda F., D'Agata F., Sacco K., Duca S., Cocito D., Paolasso I., Isoardo G., Geminiani G. Altered resting state attentional networks in diabetic neuropathic pain. Journal of Neurology, Neurosurgery, and Psychiatry. 2010;81:806–811. doi: 10.1136/jnnp.2009.188631. 19955113 [DOI] [PubMed] [Google Scholar]
- Claar R.L., Walker L.S. Functional assessment of pediatric pain patients: psychometric properties of the functional disability inventory. Pain. 2006;121:77–84. doi: 10.1016/j.pain.2005.12.002. 16480823 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen H.E., Hall J., Harris N., McCabe C.S., Blake D.R., Jänig W. Enhanced pain and autonomic responses to ambiguous visual stimuli in chronic complex regional pain syndrome (CRPS) type I. European Journal of Pain (London, England) 2012;16:182–195. doi: 10.1016/j.ejpain.2011.06.016. 22323371 [DOI] [PubMed] [Google Scholar]
- Cole M.W., Reynolds J.R., Power J.D., Repovs G., Anticevic A., Braver T.S. Multi-task connectivity reveals flexible hubs for adaptive task control. Nature Neuroscience. 2013;16:1348–1355. doi: 10.1038/nn.3470. 23892552 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cossins L., Okell R.W., Cameron H., Simpson B., Poole H.M., Goebel A. Treatment of complex regional pain syndrome in adults: a systematic review of randomized controlled trials published from June 2000 to February 2012. European Journal of Pain (London, England) 2013;17:158–173. doi: 10.1002/j.1532-2149.2012.00217.x. 23042687 [DOI] [PubMed] [Google Scholar]
- Cruz N., O'Reilly J., Slomine B.S., Salorio C.F. Emotional and neuropsychological profiles of children with complex regional pain syndrome type-I in an inpatient rehabilitation setting. Clinical Journal of Pain. 2011;27:27–34. doi: 10.1097/AJP.0b013e3181f15d95. 20842016 [DOI] [PubMed] [Google Scholar]
- Damoiseaux J.S., Rombouts S.A., Barkhof F., Scheltens P., Stam C.J., Smith S.M., Beckmann C.F. Consistent resting-state networks across healthy subjects. Proceedings of the National Academy of Sciences of the United States of America. 2006;103:13848–13853. doi: 10.1073/pnas.0601417103. 16945915 [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Jong J.R., Vlaeyen J.W., de Gelder J.M., Patijn J. Pain-related fear, perceived harmfulness of activities, and functional limitations in complex regional pain syndrome type I. Journal of Pain: Official Journal of the American Pain Society. 2011;12:1209–1218. doi: 10.1016/j.jpain.2011.06.010. 22033012 [DOI] [PubMed] [Google Scholar]
- de Jong J.R., Vlaeyen J.W., Onghena P., Cuypers C., den Hollander M., Ruijgrok J. Reduction of pain-related fear in complex regional pain syndrome type I: the application of graded exposure in vivo. Pain. 2005;116:264–275. doi: 10.1016/j.pain.2005.04.019. 15964686 [DOI] [PubMed] [Google Scholar]
- De Luca M., Beckmann C.F., De Stefano N., Matthews P.M., Smith S.M. fMRI resting state networks define distinct modes of long-distance interactions in the human brain. NeuroImage. 2006;29:1359–1367. doi: 10.1016/j.neuroimage.2005.08.035. 16260155 [DOI] [PubMed] [Google Scholar]
- Deere K.C., Clinch J., Holliday K., McBeth J., Crawley E.M., Sayers A., Palmer S., Doerner R., Clark E.M., Tobias J.H. Obesity is a risk factor for musculoskeletal pain in adolescents: findings from a population-based cohort. Pain. 2012;153:1932–1938. doi: 10.1016/j.pain.2012.06.006. 22805779 [DOI] [PubMed] [Google Scholar]
- Duke Han S.D., Buchman A.S., Arfanakis K., Fleischman D.A., Bennett D.A. Functional connectivity networks associated with chronic musculoskeletal pain in old age. International Journal of Geriatric Psychiatry. 2013;28:858–867. doi: 10.1002/gps.3898. 23124844 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fair D.A., Cohen A.L., Dosenbach N.U., Church J.A., Miezin F.M., Barch D.M., Raichle M.E., Petersen S.E., Schlaggar B.L. The maturing architecture of the brain's default network. Proceedings of the National Academy of Sciences of the United States of America. 2008;105:4028–4032. doi: 10.1073/pnas.0800376105. 18322013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Filippi M., Agosta F., Spinelli E.G., Rocca M.A. Imaging resting state brain function in multiple sclerosis. Journal of Neurology. 2013;260:1709–1713. doi: 10.1007/s00415-012-6695-z. 23052604 [DOI] [PubMed] [Google Scholar]
- Filippini N., MacIntosh B.J., Hough M.G., Goodwin G.M., Frisoni G.B., Smith S.M., Matthews P.M., Beckmann C.F., Mackay C.E. Distinct patterns of brain activity in young carriers of the APOE-epsilon4 allele. Proceedings of the National Academy of Sciences of the United States of America. 2009;106:7209–7214. doi: 10.1073/pnas.0811879106. 19357304 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fornito A., Bullmore E.T. Connectomic intermediate phenotypes for psychiatric disorders. Frontiers in Psychiatry. 2012;3:32. doi: 10.3389/fpsyt.2012.00032. 22529823 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fox M.D., Raichle M.E. Spontaneous fluctuations in brain activity observed with functional magnetic resonance imaging. Nature Reviews. Neuroscience. 2007;8:700–711. doi: 10.1038/nrn2201. 17704812 [DOI] [PubMed] [Google Scholar]
- Fox M.D., Greicius M. Clinical applications of resting state functional connectivity. Frontiers in Systems Neuroscience. 2010;4:19. doi: 10.3389/fnsys.2010.00019. 20592951 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fox M.D., Snyder A.Z., Vincent J.L., Corbetta M., Van Essen D.C., Raichle M.E. The human brain is intrinsically organized into dynamic, anticorrelated functional networks. Proceedings of the National Academy of Sciences of the United States of America. 2005;102:9673–9678. doi: 10.1073/pnas.0504136102. 15976020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frettlöh J., Hüppe M., Maier C. Severity and specificity of neglect-like symptoms in patients with complex regional pain syndrome (CRPS) compared to chronic limb pain of other origins. Pain. 2006;124:184–189. doi: 10.1016/j.pain.2006.04.010. 16730904 [DOI] [PubMed] [Google Scholar]
- Gabel C.P., Melloh M., Burkett B., Michener L.A. Lower limb functional index: development and clinimetric properties. Physical Therapy. 2012;92:98–110. doi: 10.2522/ptj.20100199. 22052947 [DOI] [PubMed] [Google Scholar]
- Galer B.S., Jensen M. Neglect-like symptoms in complex regional pain syndrome: results of a self-administered survey. Journal of Pain and Symptom Management. 1999;18:213–217. doi: 10.1016/s0885-3924(99)00076-7. 10517043 [DOI] [PubMed] [Google Scholar]
- Galer B.S., Butler S., Jensen M.P. Case reports and hypothesis: a neglect-like syndrome may be responsible for the motor disturbance in reflex sympathetic dystrophy (complex regional pain syndrome-1) Journal of Pain and Symptom Management. 1995;10:385–391. doi: 10.1016/0885-3924(95)00061-3. 7673771 [DOI] [PubMed] [Google Scholar]
- Geha P.Y., Baliki M.N., Harden R.N., Bauer W.R., Parrish T.B., Apkarian A.V. The brain in chronic CRPS pain: abnormal gray-white matter interactions in emotional and autonomic regions. Neuron. 2008;60:570–581. doi: 10.1016/j.neuron.2008.08.022. 19038215 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gierthmühlen J., Maier C., Baron R., Tölle T., Treede R.D., Birbaumer N., Huge V., Koroschetz J., Krumova E.K., Lauchart M., Maihöfner C., Richter H., Westermann A., German Research Network on Neuropathic Pain Sensory signs in complex regional pain syndrome and peripheral nerve injury. Pain. 2012;153:765–774. doi: 10.1016/j.pain.2011.11.009. 22154921 [DOI] [PubMed] [Google Scholar]
- Göttlich M., Münte T.F., Heldmann M., Kasten M., Hagenah J., Krämer U.M. Altered resting state brain networks in Parkinson's disease. PloS One. 2013;8:e77336. doi: 10.1371/journal.pone.0077336. 24204812 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goulden N., Khusnulina A., Davis N.J., Bracewell R.M., Bokde A.L., McNulty J.P., Mullins P.G. The salience network is responsible for switching between the default mode network and the central executive network: replication from DCM. NeuroImage. 2014;99:180–190. doi: 10.1016/j.neuroimage.2014.05.052. 24862074 [DOI] [PubMed] [Google Scholar]
- Greicius M.D., Flores B.H., Menon V., Glover G.H., Solvason H.B., Kenna H., Reiss A.L., Schatzberg A.F. Resting-state functional connectivity in major depression: abnormally increased contributions from subgenual cingulate cortex and thalamus. Biological Psychiatry. 2007;62:429–437. doi: 10.1016/j.biopsych.2006.09.020. 17210143 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hashmi J.A., Kong J., Spaeth R., Khan S., Kaptchuk T.J., Gollub R.L. Functional network architecture predicts psychologically mediated analgesia related to treatment in chronic knee pain patients. Journal of Neuroscience: the Official Journal of the Society for Neuroscience. 2014;34:3924–3936. doi: 10.1523/JNEUROSCI.3155-13.2014. 24623770 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoff G.E., Van den Heuvel M.P., Benders M.J., Kersbergen K.J., De Vries L.S. On development of functional brain connectivity in the young brain. Frontiers in Human Neuroscience. 2013;7:650. doi: 10.3389/fnhum.2013.00650. 24115929 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huge V., Lauchart M., Magerl W., Beyer A., Moehnle P., Kaufhold W., Schelling G., Azad S.C. Complex interaction of sensory and motor signs and symptoms in chronic CRPS. PloS One. 2011;6:e18775. doi: 10.1371/journal.pone.0018775. 21559525 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ichesco E., Schmidt-Wilcke T., Bhavsar R., Clauw D.J., Peltier S.J., Kim J., Napadow V., Hampson J.P., Kairys A.E., Williams D.A., Harris R.E. Altered resting state connectivity of the insular cortex in individuals with fibromyalgia. Journal of Pain: Official Journal of the American Pain Society. 2014;15:815–826. doi: 10.1016/j.jpain.2014.04.007. 24815079 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kachko L., Efrat R., Ben Ami S., Mukamel M., Katz J. Complex regional pain syndromes in children and adolescents. Pediatrics International: Official Journal of the Japan Pediatric Society. 2008;50:523–527. doi: 10.1111/j.1442-200X.2008.02625.x. 19143976 [DOI] [PubMed] [Google Scholar]
- Karbasforoushan H., Woodward N.D. Resting-state networks in schizophrenia. Current Topics in Medicinal Chemistry. 2012;12:2404–2414. doi: 10.2174/156802612805289863. 23279179 [DOI] [PubMed] [Google Scholar]
- Khalili-Mahani N., Zoethout R.M., Beckmann C.F., Baerends E., de Kam M.L., Soeter R.P., Dahan A., van Buchem M.A., van Gerven J.M., Rombouts S.A. Effects of morphine and alcohol on functional brain connectivity during “resting state”: a placebo-controlled crossover study in healthy young men. Human Brain Mapping. 2012;33:1003–1018. doi: 10.1002/hbm.21265. 21391283 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kilpatrick L.A., Kutch J.J., Tillisch K., Naliboff B.D., Labus J.S., Jiang Z., Farmer M.A., Apkarian A.V., Mackey S., Martucci K.T., Clauw D.J., Harris R.E., Deutsch G., Ness T.J., Yang C.C., Maravilla K., Mullins C., Mayer E.A. Alterations in resting state oscillations and connectivity in sensory and motor networks in women with interstitial cystitis/painful bladder syndrome. Journal of Urology. 2014 doi: 10.1016/j.juro.2014.03.093. 24681331 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim J.Y., Kim S.H., Seo J., Kim S.H., Han S.W., Nam E.J., Kim S.K., Lee H.J., Lee S.J., Kim Y.T., Chang Y. Increased power spectral density in resting-state pain-related brain networks in fibromyalgia. Pain. 2013;154:1792–1797. doi: 10.1016/j.pain.2013.05.040. 23714266 [DOI] [PubMed] [Google Scholar]
- Kirveskari E., Vartiainen N.V., Gockel M., Forss N. Motor cortex dysfunction in complex regional pain syndrome. Clinical Neurophysiology: Official Journal of the International Federation of Clinical Neurophysiology. 2010;121:1085–1091. doi: 10.1016/j.clinph.2010.01.032. 20185362 [DOI] [PubMed] [Google Scholar]
- Kornelsen J., Sboto-Frankenstein U., McIver T., Gervai P., Wacnik P., Berrington N., Tomanek B. Default mode network functional connectivity altered in failed back surgery syndrome. Journal of Pain: Official Journal of the American Pain Society. 2013;14:483–491. doi: 10.1016/j.jpain.2012.12.018. 23498869 [DOI] [PubMed] [Google Scholar]
- Lebel A., Becerra L., Wallin D., Moulton E.A., Morris S., Pendse G., Jasciewicz J., Stein M., Aiello-Lammens M., Grant E., Berde C., Borsook D. fMRI reveals distinct CNS processing during symptomatic and recovered complex regional pain syndrome in children. Brain: A Journal of Neurology. 2008;131:1854–1879. doi: 10.1093/brain/awn123. 18567621 [DOI] [PubMed] [Google Scholar]
- Lee M.H., Smyser C.D., Shimony J.S. Resting-state fMRI: a review of methods and clinical applications. AJNR. American Journal of Neuroradiology. 2013;34:1866–1872. doi: 10.3174/ajnr.A3263. 22936095 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li J., Zhang J.H., Yi T., Tang W.J., Wang S.W., Dong J.C. Acupuncture treatment of chronic low back pain reverses an abnormal brain default mode network in correlation with clinical pain relief. Acupuncture in Medicine: Journal of the British Medical Acupuncture Society. 2014;32:102–108. doi: 10.1136/acupmed-2013-010423. 24280949 [DOI] [PubMed] [Google Scholar]
- Libon D.J., Schwartzman R.J., Eppig J., Wambach D., Brahin E., Peterlin B.L., Alexander G., Kalanuria A. Neuropsychological deficits associated with complex regional pain syndrome. Journal of the International Neuropsychological Society: JINS. 2010;16:566–573. doi: 10.1017/S1355617710000214. 20298641 [DOI] [PubMed] [Google Scholar]
- Linnman C., Becerra L., Lebel A., Berde C., Grant P.E., Borsook D. Transient and persistent pain induced connectivity alterations in pediatric complex regional pain syndrome. PloS One. 2013;8:e57205. doi: 10.1371/journal.pone.0057205. 23526938 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Logan D.E., Carpino E.A., Chiang G., Condon M., Firn E., Gaughan V.J., Hogan M., Leslie D.S., Olson K., Sager S., Sethna N., Simons L.E., Zurakowski D., Berde C.B. A day-hospital approach to treatment of pediatric complex regional pain syndrome: initial functional outcomes. Clinical Journal of Pain. 2012;28:766–774. doi: 10.1097/AJP.0b013e3182457619. 22688602 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loggia M.L., Kim J., Gollub R.L., Vangel M.G., Kirsch I., Kong J., Wasan A.D., Napadow V. Default mode network connectivity encodes clinical pain: an arterial spin labeling study. Pain. 2013;154:24–33. doi: 10.1016/j.pain.2012.07.029. 23111164 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Low A.K., Ward K., Wines A.P. Pediatric complex regional pain syndrome. Journal of Pediatric Orthopedics. 2007;27:567–572. doi: 10.1097/BPO.0b013e318070cc4d. 17585269 [DOI] [PubMed] [Google Scholar]
- Maihöfner C., DeCol R. Decreased perceptual learning ability in complex regional pain syndrome. European Journal of Pain (London, England) 2007;11:903–909. doi: 10.1016/j.ejpain.2007.03.006. 17451979 [DOI] [PubMed] [Google Scholar]
- Maihöfner C., Birklein F. [Complex regional pain syndromes: new aspects on pathophysiology and therapy] Fortschritte Der Neurologie-Psychiatrie. 2007;75:331–342. doi: 10.1055/s-2006-944310. 17443440 [DOI] [PubMed] [Google Scholar]
- Maihöfner C., Peltz E. CRPS, the parietal cortex and neurocognitive dysfunction: an emerging triad. Pain. 2011;152:1453–1454. doi: 10.1016/j.pain.2011.03.018. 21458161 [DOI] [PubMed] [Google Scholar]
- Maihöfner C., Handwerker H.O., Birklein F. Functional imaging of allodynia in complex regional pain syndrome. Neurology. 2006;66:711–717. doi: 10.1212/01.wnl.0000200961.49114.39. 16534108 [DOI] [PubMed] [Google Scholar]
- Maihöfner C., Handwerker H.O., Neundörfer B., Birklein F. Cortical reorganization during recovery from complex regional pain syndrome. Neurology. 2004;63:693–701. doi: 10.1212/01.wnl.0000134661.46658.b0. 15326245 [DOI] [PubMed] [Google Scholar]
- Maihöfner C., Baron R., DeCol R., Binder A., Birklein F., Deuschl G., Handwerker H.O., Schattschneider J. The motor system shows adaptive changes in complex regional pain syndrome. Brain: A Journal of Neurology. 2007;130:2671–2687. doi: 10.1093/brain/awm131. 17575278 [DOI] [PubMed] [Google Scholar]
- Maldjian J.A., Laurienti P.J., Kraft R.A., Burdette J.H. An automated method for neuroanatomic and cytoarchitectonic atlas-based interrogation of fMRI data sets. NeuroImage. 2003;19:1233–1239. doi: 10.1016/s1053-8119(03)00169-1. 12880848 [DOI] [PubMed] [Google Scholar]
- Maleki J., LeBel A.A., Bennett G.J., Schwartzman R.J. Patterns of spread in complex regional pain syndrome, type I (reflex sympathetic dystrophy) Pain. 2000;88:259–266. doi: 10.1016/S0304-3959(00)00332-8. 11068113 [DOI] [PubMed] [Google Scholar]
- March J.S., Parker J.D., Sullivan K., Stallings P., Conners C.K. The Multidimensional Anxiety Scale for Children (MASC): factor structure, reliability, and validity. Journal of the American Academy of Child and Adolescent Psychiatry. 1997;36:554–565. doi: 10.1097/00004583-199704000-00019. 9100431 [DOI] [PubMed] [Google Scholar]
- Marinus J., Moseley G.L., Birklein F., Baron R., Maihöfner C., Kingery W.S., van Hilten J.J. Clinical features and pathophysiology of complex regional pain syndrome. Lancet Neurology. 2011;10:637–648. doi: 10.1016/S1474-4422(11)70106-5. 21683929 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCabe C., Mishor Z. Antidepressant medications reduce subcortical–cortical resting-state functional connectivity in healthy volunteers. NeuroImage. 2011;57:1317–1323. doi: 10.1016/j.neuroimage.2011.05.051. 21640839 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCabe C.S., Haigh R.C., Blake D.R. Mirror visual feedback for the treatment of complex regional pain syndrome (type 1) Current Pain and Headache Reports. 2008;12:103–107. doi: 10.1007/s11916-008-0020-7. 18474189 [DOI] [PubMed] [Google Scholar]
- Meindl T., Teipel S., Elmouden R., Mueller S., Koch W., Dietrich O., Coates U., Reiser M., Glaser C. Test–retest reproducibility of the default-mode network in healthy individuals. Human Brain Mapping. 2010;31:237–246. doi: 10.1002/hbm.20860. 19621371 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meng C., Brandl F., Tahmasian M., Shao J., Manoliu A., Scherr M., Schwerthöffer D., Bäuml J., Förstl H., Zimmer C., Wohlschläger A.M., Riedl V., Sorg C. Aberrant topology of striatum's connectivity is associated with the number of episodes in depression. Brain: A Journal of Neurology. 2014;137:598–609. doi: 10.1093/brain/awt290. 24163276 [DOI] [PubMed] [Google Scholar]
- Menon V., Uddin L.Q. Saliency, switching, attention and control: a network model of insula function. Brain Structure & Function. 2010;214:655–667. doi: 10.1007/s00429-010-0262-0. 20512370 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moseley G.L., Zalucki N., Birklein F., Marinus J., van Hilten J.J., Luomajoki H. Thinking about movement hurts: the effect of motor imagery on pain and swelling in people with chronic arm pain. Arthritis and Rheumatism. 2008;59:623–631. doi: 10.1002/art.23580. 18438892 [DOI] [PubMed] [Google Scholar]
- Moussa M.N., Vechlekar C.D., Burdette J.H., Steen M.R., Hugenschmidt C.E., Laurienti P.J. Changes in cognitive state alter human functional brain networks. Frontiers in Human Neuroscience. 2011;5:83. doi: 10.3389/fnhum.2011.00083. 21991252 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagler R.M. CRPS: central aspects related to locus of pain, pathophysiology, and mood. Neurology. 2010;75:109–110. doi: 10.1212/WNL.0b013e3181e7cae3. 20625163 [DOI] [PubMed] [Google Scholar]
- Napadow V., LaCount L., Park K., As-Sanie S., Clauw D.J., Harris R.E. Intrinsic brain connectivity in fibromyalgia is associated with chronic pain intensity. Arthritis and Rheumatism. 2010;62:2545–2555. doi: 10.1002/art.27497. 20506181 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pannekoek J.N., van der Werff S.J., Meens P.H., van den Bulk B.G., Jolles D.D., Veer I.M., van Lang N.D., Rombouts S.A., van der Wee N.J., Vermeiren R.R. Aberrant resting-state functional connectivity in limbic and salience networks in treatment-naive clinically depressed adolescents. Journal of Child Psychology and Psychiatry, and Allied Disciplines. 2014 doi: 10.1111/jcpp.12266. 24828372 [DOI] [PubMed] [Google Scholar]
- Patriat R., Molloy E.K., Meier T.B., Kirk G.R., Nair V.A., Meyerand M.E., Prabhakaran V., Birn R.M. The effect of resting condition on resting-state fMRI reliability and consistency: a comparison between resting with eyes open, closed, and fixated. NeuroImage. 2013;78:463–473. doi: 10.1016/j.neuroimage.2013.04.013. 23597935 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peltz E., Seifert F., Lanz S., Müller R., Maihöfner C. Impaired hand size estimation in CRPS. Journal of Pain: Official Journal of the American Pain Society. 2011;12:1095–1101. doi: 10.1016/j.jpain.2011.05.001. 21741321 [DOI] [PubMed] [Google Scholar]
- Pendse G., Borsook D., Becerra L. Enhanced false discovery rate using Gaussian mixture models for thresholding fMRI statistical maps. NeuroImage. 2009;47:231–261. doi: 10.1016/j.neuroimage.2009.02.035. 19269334 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Posner J., Hellerstein D.J., Gat I., Mechling A., Klahr K., Wang Z., McGrath P.J., Stewart J.W., Peterson B.S. Antidepressants normalize the default mode network in patients with dysthymia. JAMA Psychiatry. 2013;70:373–382. doi: 10.1001/jamapsychiatry.2013.455. 23389382 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poston K.L., Eidelberg D. Functional brain networks and abnormal connectivity in the movement disorders. NeuroImage. 2012;62:2261–2270. doi: 10.1016/j.neuroimage.2011.12.021. 22206967 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Punt T.D., Cooper L., Hey M., Johnson M.I. Neglect-like symptoms in complex regional pain syndrome: learned nonuse by another name? Pain. 2013;154:200–203. doi: 10.1016/j.pain.2012.11.006. 23290549 [DOI] [PubMed] [Google Scholar]
- Russo A., Tessitore A., Giordano A., Corbo D., Marcuccio L., De Stefano M., Salemi F., Conforti R., Esposito F., Tedeschi G. Executive resting-state network connectivity in migraine without aura. Cephalalgia: an International Journal of Headache. 2012;32:1041–1048. doi: 10.1177/0333102412457089. 22908362 [DOI] [PubMed] [Google Scholar]
- Salomons T.V., Dunlop K., Kennedy S.H., Flint A., Geraci J., Giacobbe P., Downar J. Resting-state cortico-thalamic-striatal connectivity predicts response to dorsomedial prefrontal rTMS in major depressive disorder. Neuropsychopharmacology: Official Publication of the American College of Neuropsychopharmacology. 2014;39:488–498. doi: 10.1038/npp.2013.222. 24150516 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salvador R., Suckling J., Coleman M.R., Pickard J.D., Menon D., Bullmore E. Neurophysiological architecture of functional magnetic resonance images of human brain. Cerebral Cortex (New York, N.Y.: 1991) 2005;15:1332–1342. doi: 10.1093/cercor/bhi016. 15635061 [DOI] [PubMed] [Google Scholar]
- Scheidegger M., Walter M., Lehmann M., Metzger C., Grimm S., Boeker H., Boesiger P., Henning A., Seifritz E. Ketamine decreases resting state functional network connectivity in healthy subjects: implications for antidepressant drug action. PloS One. 2012;7:e44799. doi: 10.1371/journal.pone.0044799. 23049758 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seeley W.W., Menon V., Schatzberg A.F., Keller J., Glover G.H., Kenna H., Reiss A.L., Greicius M.D. Dissociable intrinsic connectivity networks for salience processing and executive control. Journal of Neuroscience: the Official Journal of the Society for Neuroscience. 2007;27:2349–2356. doi: 10.1523/JNEUROSCI.5587-06.2007. 17329432 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seifert F., Maihöfner C. Central mechanisms of experimental and chronic neuropathic pain: findings from functional imaging studies. Cellular and Molecular Life Sciences: CMLS. 2009;66:375–390. doi: 10.1007/s00018-008-8428-0. 18791842 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Selemon L.D. A role for synaptic plasticity in the adolescent development of executive function. Translational Psychiatry. 2013;3:e238. doi: 10.1038/tp.2013.7. 23462989 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seo J., Kim S.H., Kim Y.T., Song H.J., Lee J.J., Kim S.H., Han S.W., Nam E.J., Kim S.K., Lee H.J., Lee S.J., Chang Y. Working memory impairment in fibromyalgia patients associated with altered frontoparietal memory network. PloS One. 2012;7:e37808. doi: 10.1371/journal.pone.0037808. 22715371 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sethna N.F., Meier P.M., Zurakowski D., Berde C.B. Cutaneous sensory abnormalities in children and adolescents with complex regional pain syndromes. Pain. 2007;131:153–161. doi: 10.1016/j.pain.2006.12.028. 17329025 [DOI] [PubMed] [Google Scholar]
- Shehzad Z., Kelly A.M., Reiss P.T., Gee D.G., Gotimer K., Uddin L.Q., Lee S.H., Margulies D.S., Roy A.K., Biswal B.B., Petkova E., Castellanos F.X., Milham M.P. The resting brain: unconstrained yet reliable. Cerebral Cortex (New York, N.Y.: 1991) 2009;19:2209–2229. doi: 10.1093/cercor/bhn256. 19221144 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shelby G.D., Shirkey K.C., Sherman A.L., Beck J.E., Haman K., Shears A.R., Horst S.N., Smith C.A., Garber J., Walker L.S. Functional abdominal pain in childhood and long-term vulnerability to anxiety disorders. Pediatrics. 2013;132:475–482. doi: 10.1542/peds.2012-2191. 23940244 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith S.M., Fox P.T., Miller K.L., Glahn D.C., Fox P.M., Mackay C.E., Filippini N., Watkins K.E., Toro R., Laird A.R., Beckmann C.F. Correspondence of the brain's functional architecture during activation and rest. Proceedings of the National Academy of Sciences of the United States of America. 2009;106:13040–13045. doi: 10.1073/pnas.0905267106. 19620724 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith S.M., Miller K.L., Moeller S., Xu J., Auerbach E.J., Woolrich M.W., Beckmann C.F., Jenkinson M., Andersson J., Glasser M.F., Van Essen D.C., Feinberg D.A., Yacoub E.S., Ugurbil K. Temporally-independent functional modes of spontaneous brain activity. Proceedings of the National Academy of Sciences of the United States of America. 2012;109:3131–3136. doi: 10.1073/pnas.1121329109. 22323591 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smucker M.R., Craighead W.E., Craighead L.W., Green B.J. Normative and reliability data for the children's depression inventory. Journal of Abnormal Child Psychology. 1986;14:25–39. doi: 10.1007/BF00917219. 3950219 [DOI] [PubMed] [Google Scholar]
- Sridharan D., Levitin D.J., Menon V. A critical role for the right fronto-insular cortex in switching between central-executive and default-mode networks. Proceedings of the National Academy of Sciences of the United States of America. 2008;105:12569–12574. doi: 10.1073/pnas.0800005105. 18723676 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sterling M., Jull G., Wright A. The effect of musculoskeletal pain on motor activity and control. Journal of Pain: Official Journal of the American Pain Society. 2001;2:135–145. doi: 10.1054/jpai.2001.19951. 14622823 [DOI] [PubMed] [Google Scholar]
- Sumitani M., Shibata M., Iwakura T., Matsuda Y., Sakaue G., Inoue T., Mashimo T., Miyauchi S. Pathologic pain distorts visuospatial perception. Neurology. 2007;68:152–154. doi: 10.1212/01.wnl.0000250335.56958.f0. 17210898 [DOI] [PubMed] [Google Scholar]
- Tagliazucchi E., Balenzuela P., Fraiman D., Chialvo D.R. Brain resting state is disrupted in chronic back pain patients. Neuroscience Letters. 2010;485:26–31. doi: 10.1016/j.neulet.2010.08.053. 20800649 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tan E.C., van de Sandt-Renkema N., Krabbe P.F., Aronson D.C., Severijnen R.S. Quality of life in adults with childhood-onset of complex regional pain syndrome type I. Injury. 2009;40:901–904. doi: 10.1016/j.injury.2009.01.134. 19524904 [DOI] [PubMed] [Google Scholar]
- Thomason M.E., Dennis E.L., Joshi A.A., Joshi S.H., Dinov I.D., Chang C., Henry M.L., Johnson R.F., Thompson P.M., Toga A.W., Glover G.H., Van Horn J.D., Gotlib I.H. Resting-state fMRI can reliably map neural networks in children. NeuroImage. 2011;55:165–175. doi: 10.1016/j.neuroimage.2010.11.080. 21134471 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uddin L.Q., Supekar K.S., Ryali S., Menon V. Dynamic reconfiguration of structural and functional connectivity across core neurocognitive brain networks with development. Journal of Neuroscience: the Official Journal of the Society for Neuroscience. 2011;31:18578–18589. doi: 10.1523/JNEUROSCI.4465-11.2011. 22171056 [DOI] [PMC free article] [PubMed] [Google Scholar]
- van den Heuvel M.P., Hulshoff Pol H.E. Exploring the brain network: a review on resting-state fMRI functional connectivity. European Neuropsychopharmacology: the Journal of the European College of Neuropsychopharmacology. 2010;20:519–534. doi: 10.1016/j.euroneuro.2010.03.008. 20471808 [DOI] [PubMed] [Google Scholar]
- van den Heuvel M.P., Stam C.J., Boersma M., Hulshoff Pol H.E. Small-world and scale-free organization of voxel-based resting-state functional connectivity in the human brain. NeuroImage. 2008;43:528–539. doi: 10.1016/j.neuroimage.2008.08.010. 18786642 [DOI] [PubMed] [Google Scholar]
- van den Heuvel M.P., Mandl R.C., Kahn R.S., Hulshoff Pol H.E. Functionally linked resting-state networks reflect the underlying structural connectivity architecture of the human brain. Human Brain Mapping. 2009;30:3127–3141. doi: 10.1002/hbm.20737. 19235882 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verdugo R.J., Ochoa J.L. Abnormal movements in complex regional pain syndrome: assessment of their nature. Muscle & Nerve. 2000;23:198–205. doi: 10.1002/(sici)1097-4598(200002)23:2<198::aid-mus9>3.0.co;2-4. 10639611 [DOI] [PubMed] [Google Scholar]
- Walker L.S., Greene J.W. The functional disability inventory: measuring a neglected dimension of child health status. Journal of Pediatric Psychology. 1991;16:39–58. doi: 10.1093/jpepsy/16.1.39. 1826329 [DOI] [PubMed] [Google Scholar]
- Walker L.S., Dengler-Crish C.M., Rippel S., Bruehl S. Functional abdominal pain in childhood and adolescence increases risk for chronic pain in adulthood. Pain. 2010;150:568–572. doi: 10.1016/j.pain.2010.06.018. 20615615 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zuo X.N., Kelly C., Adelstein J.S., Klein D.F., Castellanos F.X., Milham M.P. Reliable intrinsic connectivity networks: test–retest evaluation using Ica and dual regression approach. NeuroImage. 2010;49:2163–2177. doi: 10.1016/j.neuroimage.2009.10.080. 19896537 [DOI] [PMC free article] [PubMed] [Google Scholar]


