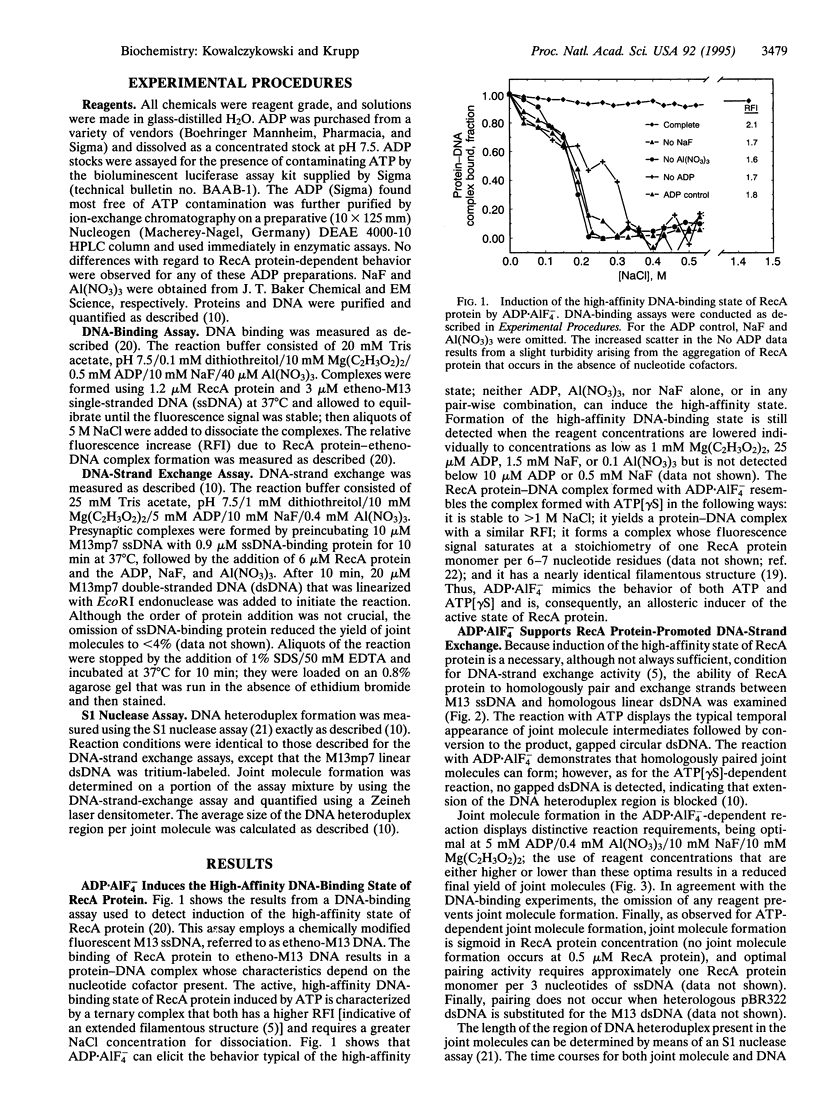
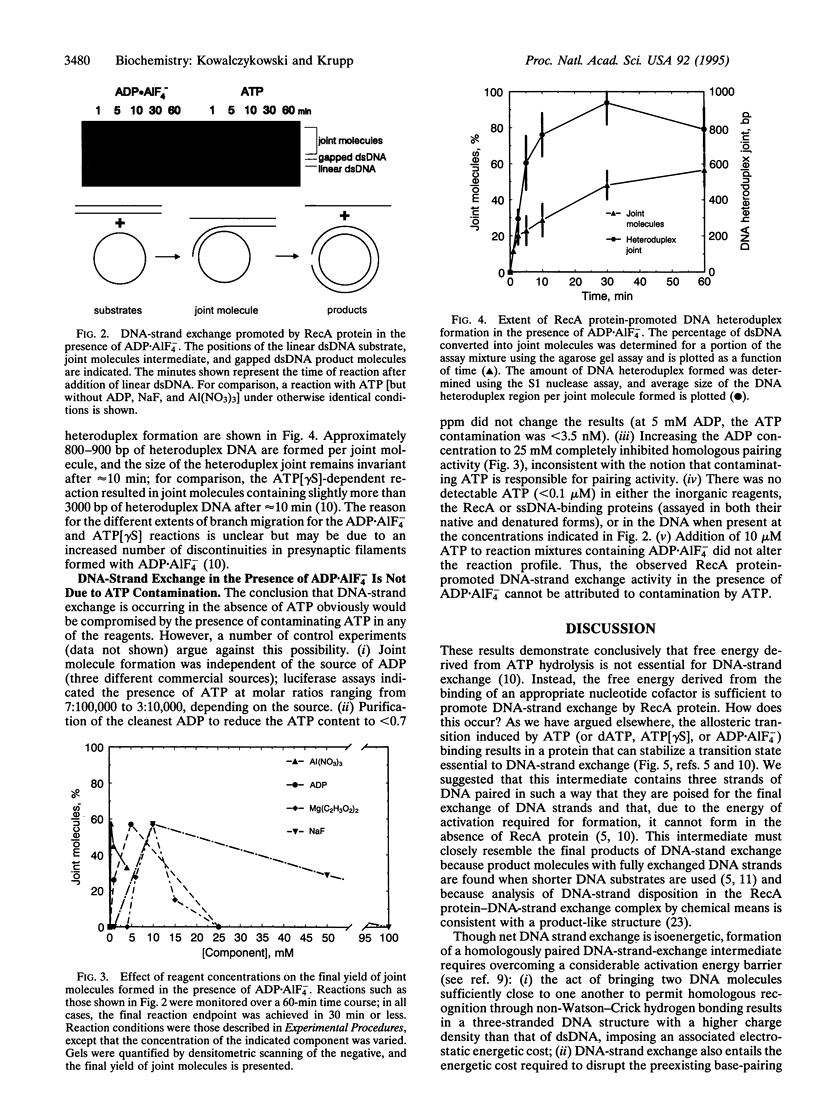
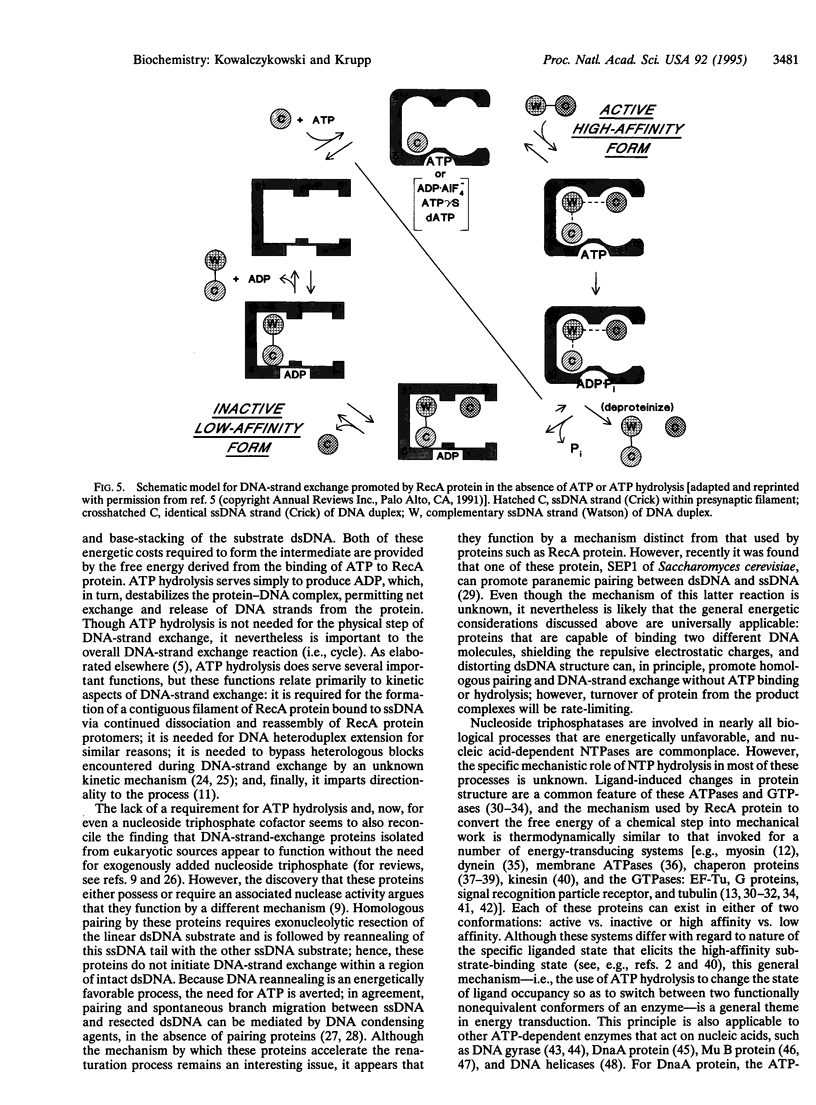
Abstract
DNA-strand exchange promoted by Escherichia coli RecA protein normally requires the presence of ATP and is accompanied by ATP hydrolysis, thereby implying a need for ATP hydrolysis. Previously, ATP hydrolysis was shown not to be required; here we demonstrate furthermore that a nucleoside triphosphate cofactor is not required for DNA-strand exchange. A gratuitous allosteric effector consisting of the noncovalent complex of ADP and aluminum fluoride, ADP.AIF4-, can both induce the high-affinity DNA-binding state of RecA protein and support the homologous pairing and exchange of up to 800-900 bp of DNA. These results demonstrate that induction of the functionally active, high-affinity DNA-binding state of RecA protein is needed for RecA protein-promoted DNA-strand exchange and that there is no requirement for a high-energy nucleotide cofactor for the exchange of DNA strands. Consequently, the free energy needed to activate the DNA substrates for DNA-strand exchange is not derived from ATP hydrolysis. Instead, the needed free energy is derived from ligand binding and is transduced to the DNA via the associated ligand-induced structural transitions of the RecA protein-DNA complex; ATP hydrolysis simply destroys the effector ligand. This concept has general applicability to the mechanism of energy transduction by proteins.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adzuma K., Mizuuchi K. Interaction of proteins located at a distance along DNA: mechanism of target immunity in the Mu DNA strand-transfer reaction. Cell. 1989 Apr 7;57(1):41–47. doi: 10.1016/0092-8674(89)90170-0. [DOI] [PubMed] [Google Scholar]
- Adzuma K., Mizuuchi K. Steady-state kinetic analysis of ATP hydrolysis by the B protein of bacteriophage mu. Involvement of protein oligomerization in the ATPase cycle. J Biol Chem. 1991 Apr 5;266(10):6159–6167. [PubMed] [Google Scholar]
- Adzuma K. Stable synapsis of homologous DNA molecules mediated by the Escherichia coli RecA protein involves local exchange of DNA strands. Genes Dev. 1992 Sep;6(9):1679–1694. doi: 10.1101/gad.6.9.1679. [DOI] [PubMed] [Google Scholar]
- Alberts B., Miake-Lye R. Unscrambling the puzzle of biological machines: the importance of the details. Cell. 1992 Feb 7;68(3):415–420. doi: 10.1016/0092-8674(92)90179-g. [DOI] [PubMed] [Google Scholar]
- Allende J. E. GTP-mediated macromolecular interactions: the common features of different systems. FASEB J. 1988 May;2(8):2356–2367. doi: 10.1096/fasebj.2.8.2452111. [DOI] [PubMed] [Google Scholar]
- Berchtold H., Reshetnikova L., Reiser C. O., Schirmer N. K., Sprinzl M., Hilgenfeld R. Crystal structure of active elongation factor Tu reveals major domain rearrangements. Nature. 1993 Sep 9;365(6442):126–132. doi: 10.1038/365126a0. [DOI] [PubMed] [Google Scholar]
- Bourne H. R., Sanders D. A., McCormick F. The GTPase superfamily: a conserved switch for diverse cell functions. Nature. 1990 Nov 8;348(6297):125–132. doi: 10.1038/348125a0. [DOI] [PubMed] [Google Scholar]
- Chabre M. Aluminofluoride and beryllofluoride complexes: a new phosphate analogs in enzymology. Trends Biochem Sci. 1990 Jan;15(1):6–10. doi: 10.1016/0968-0004(90)90117-t. [DOI] [PubMed] [Google Scholar]
- Chen J., Kanaar R., Cozzarelli N. R. The Sep1 strand exchange protein from Saccharomyces cerevisiae promotes a paranemic joint between homologous DNA molecules. Genes Dev. 1994 Jun 1;8(11):1356–1366. doi: 10.1101/gad.8.11.1356. [DOI] [PubMed] [Google Scholar]
- Cox M. M., Lehman I. R. Enzymes of general recombination. Annu Rev Biochem. 1987;56:229–262. doi: 10.1146/annurev.bi.56.070187.001305. [DOI] [PubMed] [Google Scholar]
- Cox M. M., Lehman I. R. recA protein of Escherichia coli promotes branch migration, a kinetically distinct phase of DNA strand exchange. Proc Natl Acad Sci U S A. 1981 Jun;78(6):3433–3437. doi: 10.1073/pnas.78.6.3433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eggleston A. K., Kowalczykowski S. C. An overview of homologous pairing and DNA strand exchange proteins. Biochimie. 1991 Feb-Mar;73(2-3):163–176. doi: 10.1016/0300-9084(91)90199-b. [DOI] [PubMed] [Google Scholar]
- Flynn G. C., Chappell T. G., Rothman J. E. Peptide binding and release by proteins implicated as catalysts of protein assembly. Science. 1989 Jul 28;245(4916):385–390. doi: 10.1126/science.2756425. [DOI] [PubMed] [Google Scholar]
- Hill T. L. A proposed common allosteric mechanism for active transport, muscle contraction, and ribosomal translocation. Proc Natl Acad Sci U S A. 1969 Sep;64(1):267–274. doi: 10.1073/pnas.64.1.267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jencks W. P. How does a calcium pump pump calcium? J Biol Chem. 1989 Nov 15;264(32):18855–18858. [PubMed] [Google Scholar]
- Johnson K. A. Pathway of the microtubule-dynein ATPase and the structure of dynein: a comparison with actomyosin. Annu Rev Biophys Biophys Chem. 1985;14:161–188. doi: 10.1146/annurev.bb.14.060185.001113. [DOI] [PubMed] [Google Scholar]
- Kim J. I., Cox M. M., Inman R. B. On the role of ATP hydrolysis in RecA protein-mediated DNA strand exchange. I. Bypassing a short heterologous insert in one DNA substrate. J Biol Chem. 1992 Aug 15;267(23):16438–16443. [PubMed] [Google Scholar]
- Kmiec E. B., Holloman W. K. DNA strand exchange in the absence of homologous pairing. J Biol Chem. 1994 Apr 1;269(13):10163–10168. [PubMed] [Google Scholar]
- Kowalczykowski S. C. Biochemistry of genetic recombination: energetics and mechanism of DNA strand exchange. Annu Rev Biophys Biophys Chem. 1991;20:539–575. doi: 10.1146/annurev.bb.20.060191.002543. [DOI] [PubMed] [Google Scholar]
- Kowalczykowski S. C., Dixon D. A., Eggleston A. K., Lauder S. D., Rehrauer W. M. Biochemistry of homologous recombination in Escherichia coli. Microbiol Rev. 1994 Sep;58(3):401–465. doi: 10.1128/mr.58.3.401-465.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kowalczykowski S. C., Eggleston A. K. Homologous pairing and DNA strand-exchange proteins. Annu Rev Biochem. 1994;63:991–1043. doi: 10.1146/annurev.bi.63.070194.005015. [DOI] [PubMed] [Google Scholar]
- Lauder S. D., Kowalczykowski S. C. Asymmetry in the recA protein-DNA filament. J Biol Chem. 1991 Mar 25;266(9):5450–5458. [PubMed] [Google Scholar]
- Martin J., Mayhew M., Langer T., Hartl F. U. The reaction cycle of GroEL and GroES in chaperonin-assisted protein folding. Nature. 1993 Nov 18;366(6452):228–233. doi: 10.1038/366228a0. [DOI] [PubMed] [Google Scholar]
- Maruta S., Henry G. D., Sykes B. D., Ikebe M. Formation of the stable myosin-ADP-aluminum fluoride and myosin-ADP-beryllium fluoride complexes and their analysis using 19F NMR. J Biol Chem. 1993 Apr 5;268(10):7093–7100. [PubMed] [Google Scholar]
- Menetski J. P., Bear D. G., Kowalczykowski S. C. Stable DNA heteroduplex formation catalyzed by the Escherichia coli RecA protein in the absence of ATP hydrolysis. Proc Natl Acad Sci U S A. 1990 Jan;87(1):21–25. doi: 10.1073/pnas.87.1.21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Menetski J. P., Kowalczykowski S. C. Interaction of recA protein with single-stranded DNA. Quantitative aspects of binding affinity modulation by nucleotide cofactors. J Mol Biol. 1985 Jan 20;181(2):281–295. doi: 10.1016/0022-2836(85)90092-0. [DOI] [PubMed] [Google Scholar]
- Milburn M. V., Tong L., deVos A. M., Brünger A., Yamaizumi Z., Nishimura S., Kim S. H. Molecular switch for signal transduction: structural differences between active and inactive forms of protooncogenic ras proteins. Science. 1990 Feb 23;247(4945):939–945. doi: 10.1126/science.2406906. [DOI] [PubMed] [Google Scholar]
- Miller J. D., Wilhelm H., Gierasch L., Gilmore R., Walter P. GTP binding and hydrolysis by the signal recognition particle during initiation of protein translocation. Nature. 1993 Nov 25;366(6453):351–354. doi: 10.1038/366351a0. [DOI] [PubMed] [Google Scholar]
- Moreau P. L., Carlier M. F. RecA protein-promoted cleavage of LexA repressor in the presence of ADP and structural analogues of inorganic phosphate, the fluoride complexes of aluminum and beryllium. J Biol Chem. 1989 Feb 5;264(4):2302–2306. [PubMed] [Google Scholar]
- Osheroff N. Eukaryotic topoisomerase II. Characterization of enzyme turnover. J Biol Chem. 1986 Jul 25;261(21):9944–9950. [PubMed] [Google Scholar]
- Pai E. F., Kabsch W., Krengel U., Holmes K. C., John J., Wittinghofer A. Structure of the guanine-nucleotide-binding domain of the Ha-ras oncogene product p21 in the triphosphate conformation. Nature. 1989 Sep 21;341(6239):209–214. doi: 10.1038/341209a0. [DOI] [PubMed] [Google Scholar]
- Palleros D. R., Reid K. L., Shi L., Welch W. J., Fink A. L. ATP-induced protein-Hsp70 complex dissociation requires K+ but not ATP hydrolysis. Nature. 1993 Oct 14;365(6447):664–666. doi: 10.1038/365664a0. [DOI] [PubMed] [Google Scholar]
- Peebles C. L., Higgins N. P., Kreuzer K. N., Morrison A., Brown P. O., Sugino A., Cozzarelli N. R. Structure and activities of Escherichia coli DNA gyrase. Cold Spring Harb Symp Quant Biol. 1979;43(Pt 1):41–52. doi: 10.1101/sqb.1979.043.01.008. [DOI] [PubMed] [Google Scholar]
- Phan B. C., Faller L. D., Reisler E. Kinetic and equilibrium analysis of the interactions of actomyosin subfragment-1.ADP with beryllium fluoride. Biochemistry. 1993 Aug 3;32(30):7712–7719. doi: 10.1021/bi00081a016. [DOI] [PubMed] [Google Scholar]
- Radding C. M. Helical interactions in homologous pairing and strand exchange driven by RecA protein. J Biol Chem. 1991 Mar 25;266(9):5355–5358. [PubMed] [Google Scholar]
- Romberg L., Vale R. D. Chemomechanical cycle of kinesin differs from that of myosin. Nature. 1993 Jan 14;361(6408):168–170. doi: 10.1038/361168a0. [DOI] [PubMed] [Google Scholar]
- Rosselli W., Stasiak A. Energetics of RecA-mediated recombination reactions. Without ATP hydrolysis RecA can mediate polar strand exchange but is unable to recycle. J Mol Biol. 1990 Nov 20;216(2):335–352. doi: 10.1016/S0022-2836(05)80325-0. [DOI] [PubMed] [Google Scholar]
- Rosselli W., Stasiak A. The ATPase activity of RecA is needed to push the DNA strand exchange through heterologous regions. EMBO J. 1991 Dec;10(13):4391–4396. doi: 10.1002/j.1460-2075.1991.tb05017.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruigrok R. W., Bohrmann B., Hewat E., Engel A., Kellenberger E., DiCapua E. The inactive form of recA protein: the 'compact' structure. EMBO J. 1993 Jan;12(1):9–16. doi: 10.1002/j.1460-2075.1993.tb05626.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sancar A., Hearst J. E. Molecular matchmakers. Science. 1993 Mar 5;259(5100):1415–1420. doi: 10.1126/science.8451638. [DOI] [PubMed] [Google Scholar]
- Schlichting I., Almo S. C., Rapp G., Wilson K., Petratos K., Lentfer A., Wittinghofer A., Kabsch W., Pai E. F., Petsko G. A. Time-resolved X-ray crystallographic study of the conformational change in Ha-Ras p21 protein on GTP hydrolysis. Nature. 1990 May 24;345(6273):309–315. doi: 10.1038/345309a0. [DOI] [PubMed] [Google Scholar]
- Sekimizu K., Bramhill D., Kornberg A. ATP activates dnaA protein in initiating replication of plasmids bearing the origin of the E. coli chromosome. Cell. 1987 Jul 17;50(2):259–265. doi: 10.1016/0092-8674(87)90221-2. [DOI] [PubMed] [Google Scholar]
- Sikorav J. L., Church G. M. Complementary recognition in condensed DNA: accelerated DNA renaturation. J Mol Biol. 1991 Dec 20;222(4):1085–1108. doi: 10.1016/0022-2836(91)90595-w. [DOI] [PubMed] [Google Scholar]
- Sugino A., Higgins N. P., Brown P. O., Peebles C. L., Cozzarelli N. R. Energy coupling in DNA gyrase and the mechanism of action of novobiocin. Proc Natl Acad Sci U S A. 1978 Oct;75(10):4838–4842. doi: 10.1073/pnas.75.10.4838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor E. W. Cell motility. Variations on the theme of movement. Nature. 1993 Jan 14;361(6408):115–116. doi: 10.1038/361115a0. [DOI] [PubMed] [Google Scholar]
- Vale R. D. Getting a grip on myosin. Cell. 1994 Sep 9;78(5):733–737. doi: 10.1016/s0092-8674(94)90402-2. [DOI] [PubMed] [Google Scholar]
- West S. C. Enzymes and molecular mechanisms of genetic recombination. Annu Rev Biochem. 1992;61:603–640. doi: 10.1146/annurev.bi.61.070192.003131. [DOI] [PubMed] [Google Scholar]
- Wong I., Lohman T. M. Allosteric effects of nucleotide cofactors on Escherichia coli Rep helicase-DNA binding. Science. 1992 Apr 17;256(5055):350–355. doi: 10.1126/science.256.5055.350. [DOI] [PubMed] [Google Scholar]
- Yu X., Egelman E. H. Image analysis reveals that Escherichia coli RecA protein consists of two domains. Biophys J. 1990 Mar;57(3):555–566. doi: 10.1016/S0006-3495(90)82571-6. [DOI] [PMC free article] [PubMed] [Google Scholar]