Abstract
Objective
The aim of this study is to evaluate the clinical results of endoscopic radiofrequency ablation of medial branch in patients with chronic low back pain originating from facet joints.
Methods
Between October 2010 and December 2013, 52 consecutive patients had suffering from chronic low back pain had undergone endoscopic radiofrequency denervation of medial branch of dorsal ramus. The clinical outcomes of these 52 patients were reviewed retrospectively. Preoperative and postoperative Visual Analogue Scale (VAS) and Korean version of Oswestry Disability Index (K-ODI), and patients' satisfaction with the procedure were assessed.
Results
The pain scores on the VAS for back pain had improved significantly from a preoperative mean of 7.1 to a postoperative mean of 2 at the last follow-up (p<0.001). The clinical outcomes based on the K-ODI had also improved significantly from a preoperative mean of 26.5% to postoperative mean of 7.7% at the last follow-up (p<0.001). 80% of patients were satisfied with the procedure. There were no complications associated with the procedure.
Conclusion
Our preliminary results demonstrate that endoscopic radiofrequency denervation of medial branch could be an effective alternative treatment modality for chronic back pain originating from facet joints that provides long-term pain relief.
Keywords: Endoscopic radiofrequency, Chronic low back pain, Facet joint, Medial branch
INTRODUCTION
The lifetime prevalence of low back pain is estimated to range from 60% to 80%4). Chronic low back pain (CLBP), or low back pain that persists for 3 months or more is reported to have a lifetime prevalence of 4% to 10%8), and is associated with substantial health care costs10). Identifying the source of CLBP and selecting proper treatment for it is an issue of great concern for spine surgeons, as well as rehabilitation and pain interventional specialists.
Low back pain arising from facet joint pain, or facet joint syndrome (FJS), is a major source of CLBP and is reported to be responsible for 15% to 45% of total number of population suffering from CLBP15,17). Symptoms can be similar to those of herniated disc, and pain can be exacerbated by back extension after flexion. The current standard treatment modalities for FJS are intra-articular anesthetic steroid injection, medial branch block (MBB), and radiofrequency (RF) medial branch denervation17,19).
The intra-articular injection and MBB are easy-to-perform, non-surgical procedures with additional diagnostic values. However, patients may experience symptom recurrence due to short effective duration, and there are always risks of possible local and systemic complications associated with repeated steroids injection.
Fluoroscopy-guided RF denervation of the medial branch provides longer lasting effects than aforementioned treatment options, with comparable precision and reproducibility, and is currently preferred over other modalities. However, due to the variations in median nerve passages not only in degenerative or postoperative spine but also in normal spine, often extensive ablation is required to achieve satisfactory relief of patients' pain. Extensive ablation can also scar adjacent muscular and ligamentous structures, which in itself can become source of CLBP.
To overcome these shortcomings, we employed a novel method for RF denervation for treatment of FJS, in which with endoscopic guidance, directly visualization of medial branch was attempted prior to ablation for more precise lesioning and effective neural denervation without damaging nearby structures. We retrospectively reviewed clinical outcomes and satisfaction rates in our initial group of patients treated using this endoscopic RF denervation method.
MATERIALS AND METHODS
Patient selection
We retrospectively reviewed the clinical data of 52 consecutive patients with CLBP arising from FJS who were treated with endoscopic RF denervation of medial branch at our institution between October 2010 and December 2013. This single group of patients was comprised of 19 men and 33 women, and the mean age was 62.1±10.01 years.
To undergo endoscopic RF ablation, all patients must have had CLBP with a minimum Visual Analogue Scale (VAS) score of 7, and must have been refractory to a minimum of 2 months of conservative and medical treatment including analgesics and physical therapy. Two MBB were performed at separate occasions to rule out false positive results, and if the patient was responsive to both MBBs, endoscopic RF ablation was performed. Responsiveness to MBB was defined as 50% or more alleviation of pain. Discography was not performed for to rule out CLBP of disc origin, because the procedure has been reported to be associated with accelerated progression of disc herniation5).
Patients with definite lumbar instability prompting surgical stabilization, prominent radiating leg pain, history of concomitant scoliosis of more than 15 degrees, sagittal misalignment requiring deformity surgery, metabolic bone disease, vertebral fractures, or tumors were excluded from the study, as well as those with unresolved issues of secondary gain or worker's compensation.
Surgical technique
All procedures were performed in operating room under fluoroscopy with patient under light anesthesia using intravenous fentanyl. After verification of target level with C-arm, skin anesthesia was done at needle entry site with via 22-gauge spinal needle with 1% lidocaine. The facet joint is innervated by medial branch of dorsal ramus at the level and one level above it. Therefore, to successfully treat pain arising from a facet joint, the medial branch one level above the target needs to be ablated as well. Dorsal ramus also gives off lateral branch and sometimes intermediate branch, and while they do not primarily innervate the facet joints, they provide iliolumbar musculature and cutaneous innervation, and may contribute to generation of back pain. The target point for ablation is the junction of the transverse process and the base of the superior articular process (SAP). With C-arm in oblique position to check needle trajectory and position, an 18 G needle was inserted through the sterilized skin and docked onto target point. Anteroposterior (AP) fluoroscopic view with maximal exposure of the target was obtained and image was saved. Next, skin opening was widened slightly with No. 11 scalpel, and a K-wire, obturator, and beveled working cannula were serially inserted through the opening. After verifying the correct position of cannula under C-arm, Short Vertebris Spine Endoscope (Richard Wolf GmBH, Knittlingen, Germany) with 3.1 mm working channel was advanced through the cannula, and Trigger-flex disposable bipolar probe (Richard Wolf GmBH, Knittlingen, Germany) was advanced through the working channel in endoscope. Soft tissue at base of transverse process, including medial and lateral branch was removed. After confirmation of medial branch over the junction of transverse process and superior articular process, while maintaining continuous saline irrigation, medial branch was stimulated with RF bipolar probe tip to see if any pain concordant to the patient's usual pain is elicited. If the concordant pain was elicited, selective denervation of the branch was performed using RF probe, while maintaining continuous saline irrigation.
The endpoint of the procedure was when stimulation of previously ablated area did not elicit significant pain any further.
Pain and functional outcome assessment
All patients were assessed using the VAS (0 cm : no pain; 10 cm : worst imaginable pain) and the Korean version of Oswestry Disability Index (K-ODI). These were administered before the endoscopic RF ablation procedure, immediately after the procedure, and 6, 12, and 24 months at follow-up outpatient clinic visits. Additionally, two questions were asked at patients' final visit : patients' satisfaction with the procedure and willingness to receive treatment again if pain persists. Patient's satisfaction was assessed with a 4-point scale questionnaire, ranging from 4 points (very satisfied) to 1 point (very dissatisfied). Willingness to receive treatment again was checked in similar fashion using a 5-point scale questionnaire, ranging from 5 points (definitely will) to 1 point (definitely will not).
Statistical analysis
Mean VAS and K-ODI scores after the procedure at 6, 12, and 24 months follow-up visit were compared with those before the procedure. Paired Student t-test was employed to evaluate the statistical significance, and p<0.05 was considered to indicate a statistically significant difference. All statistical analysis was done using Statistical Package for the Social Sciences (SPSS) ver. 17.0 (SPSS, Inc., Chicago, IL, USA).
RESULTS
Patient demographics and perioperative ata
A total of 52 patients with CLBP, including 19 men and 33 women met the inclusion criteria. Mean age was 62.1 (±10.1) years, and the mean duration of pain was 24.4 (±15.8) months. Symptomatic level was at L4-5 level in 29 patients, at the L5-S1 level in 18 patients, at the L3-4 level in 14 patients, and L2-3 level at 2 patients. As for number of levels treated simultaneously, 8 patients were operated at 1 level, 14 on 2 levels, 10 on 3 levels, and 1 patient at 4 levels. The mean duration of operation was 0.6 hours. There were no transient or persistent complications associated with the procedure (Table 1).
Table 1.
Demographic, clinical and intraoperative data of patients who underwent RFA
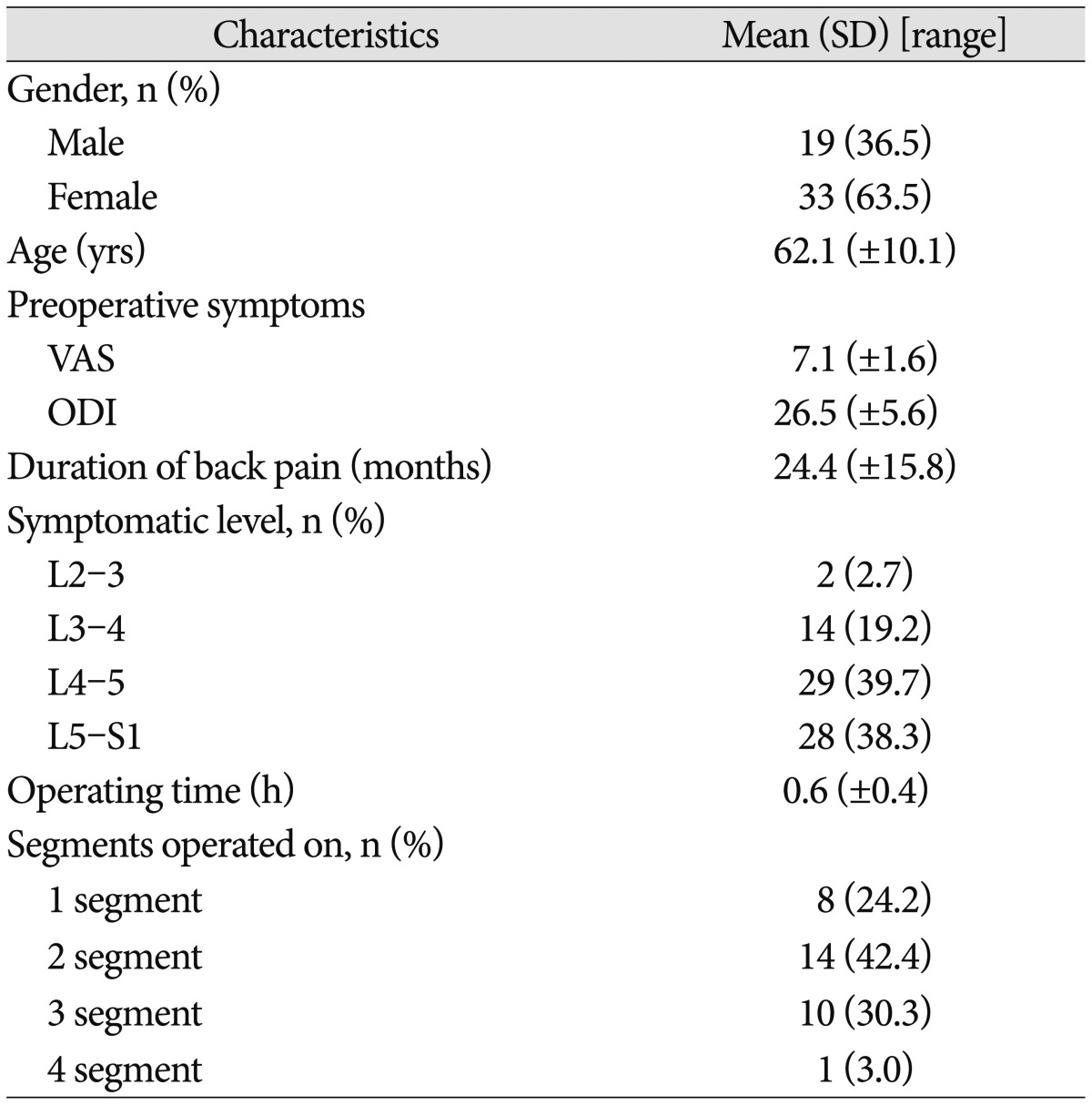
RFA : radiafrequency ablation, VAS : Visual Analogue Scale, K-ODI : Oswestry Disability Index
Clinical outcomes
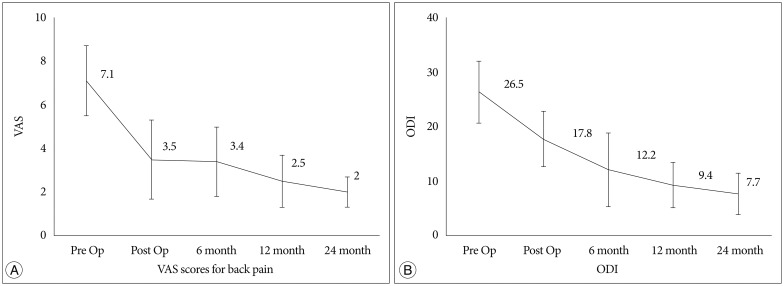
The mean preoperative VAS score for back pain was 7.1, which improved to 2.0 (p<0.001) by the last follow-up outpatient clinic visit, and the patients' mean score on the K-ODI in a similar manner improved from 26.5% preoperatively to 7.7% (p<0.001) by the last follow-up (Fig. 1). 9 of the 52 patients (17.3%) had diminished back pain but still had residual leg discomfort. Therefore, these patients underwent fluoroscopy-guided selective nerve root block after the procedure.
Fig. 1.
The mean preoperative VAS score for back pain was 7.1, which improved to 2.0 at 24 months' follow-up (p<0.001) (A), and the patients' mean score on the K-ODI improved from 26.5% to 7.7% (p<0.001) by the 24 months' follow-up (B). VAS : Visual Analogue Scale, K-ODI : Oswestry Disability Index.
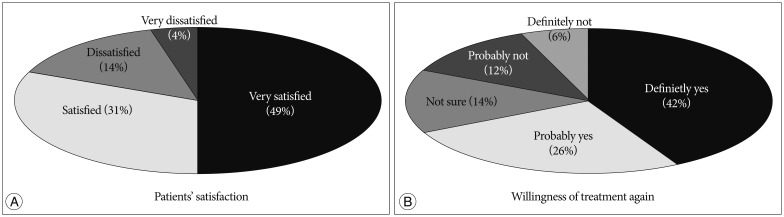
Total of 49% of the patients were very satisfied with the procedure, 31% were satisfied, 14% were dissatisfied, and 4% were very dissatisfied. As for willingness to receive same treatment again, 42% answered that they definitely would, 26% answered probably yes, 14% and 6% answered they would probably not and definitely not, respectively (Fig. 2).
Fig. 2.
Patients' satisfaction (A) and willingness to receive treatment again (B) were measured using questionnaire sheets with 4 point and 5 point scales, respectively.
Illustrative case
A 76-year-old female who had undergone posterior interbody fusion of L4-5 and L5-S1 for treatment of spinal stenosis 18 months ago suffered from CLBP on the right side that was refractory to conservative treatment with opioid analgesics. Neurological examination revealed no neurological deficit including motor weakness and cauda equina symptoms. She did not complain of radiating pain and had no evident tender point on her low back. However, her sitting intolerance was worsening and she felt worst pain on extension of her back.
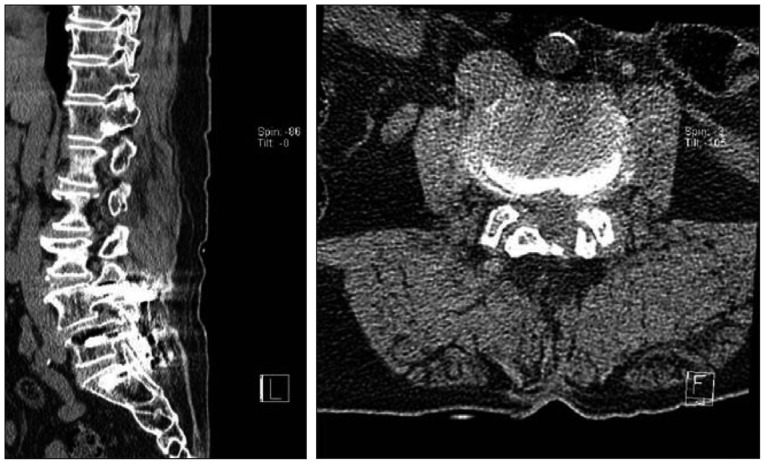
Initial VAS was 7 points and K-ODI 27%. CT revealed adjacent segment degeneration with diffuse bulging disc at L3-4 level (Fig. 3). We performed MBB of L3 and L4 medial branch on the right side, and her VAS score dropped immediately to 1 point (Fig. 4). At follow up visit 1 month later, the patient's VAS score had worsened to 5 points, and same procedure was repeated, after which VAS dropped to 1 point again.
Fig. 3.
Non-contrast computed tomography (CT) image shows adjacent segment degeneration at L3-4 with diffuse bulging disc and bilaterally hypertrophied facets.
Fig. 4.
Fluoroscopic images of L4 medial branch block. Same procedure was repeated for L3 medial branch, as L3-4 facet receives dual innervation from L3 and L4 medial branches.
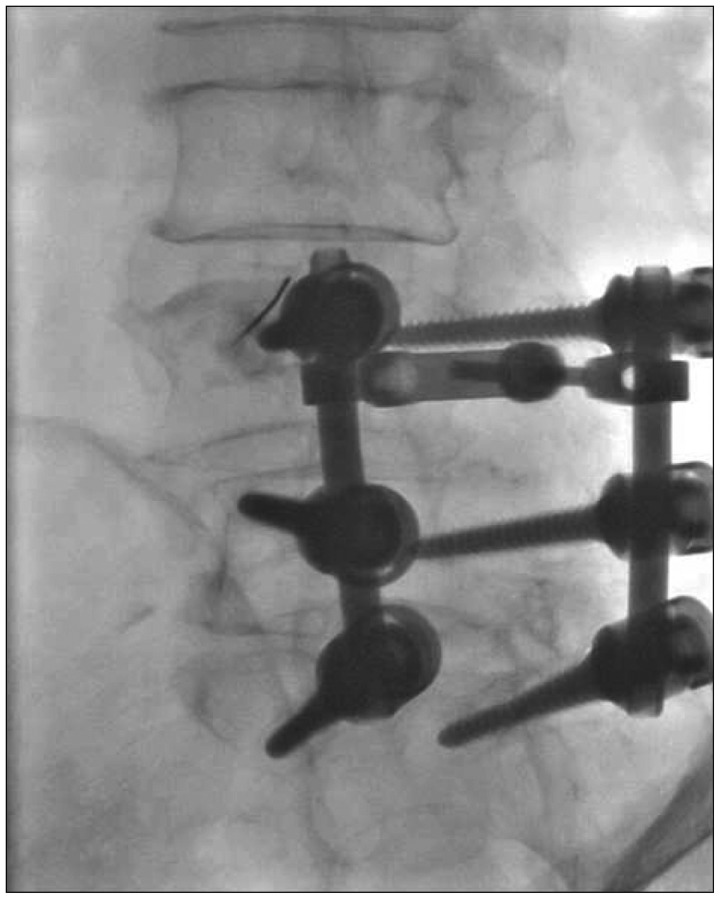
Endoscopic RF denervation of medial branch of L3 and L4 of her right side was planned and the patient underwent the procedure 1 week later (Fig. 5, 6). Medial branch of dorsal ramus was visualized under endoscope (Fig. 7) and stimulation of the nerve with RF bipolar probe elicited pain. Immediately after the procedure, the patient's VAS was 1 point and K-ODI 19%. At 6 weeks' follow up, she did not complain of worsening of symptoms, with VAS score of 2 patients and K-ODI of 20%.
Fig. 5.
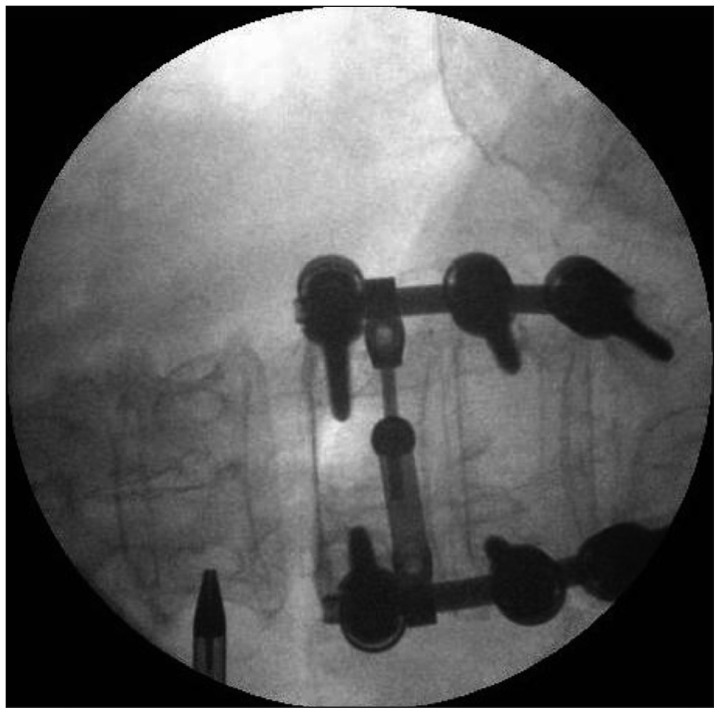
Intraprocedural fluoroscopic image showing cannula location during endoscopic RF denervation of L3 medial branch. First, 18 G needle was docked onto target point, at the junction of transverse process and superior articular process, and its position was confirmed on C-arm images. Then working cannula was inserted through the trajectory made by 18 G needle. RF : radiofrequency.
Fig. 6.
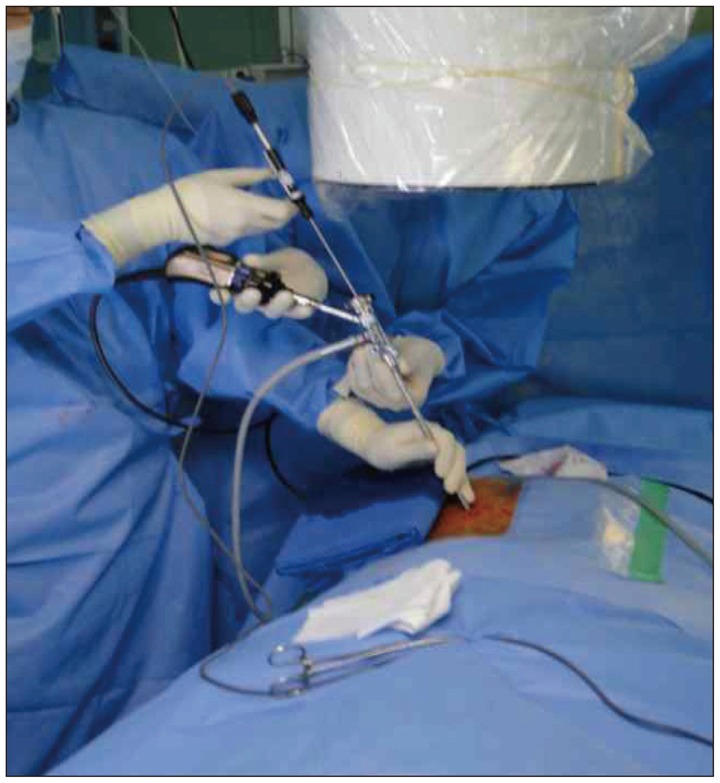
Bipolar RF probe was used to denervate target medial branch once it was identified under endoscope. RF : radiofrequency.
Fig. 7.
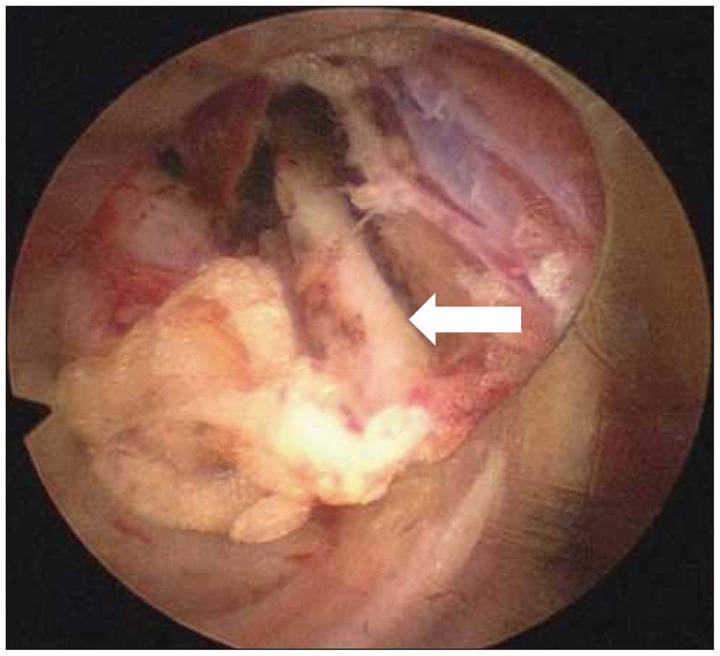
Endoscopic view shows medial branch of dorsal ramus (white arrow) as it courses caudally from the junction of transverse process and superior articular facet.
DISCUSSION
The intervertebral disc, facet joint, and sacroiliac joint are the three primary structures within the spinal component that are most common sources of CLBP7,14). In 1933, Ghormley first described the term 'facet syndrome' as a cause of referred pain and the sciatica coming from direct root compression by the facet9). Badgley1) was the first to report in great detail that the facet joint could be an independent source of referred pain, and since then many studies have been published about its clinical importance in generation of CLBP, as well as diagnostic methods and treatment methods6,12,21).
Facet joints have synovial linings and capsules and are highly innervated by free nerve endings in the tissues16). Facet joints can become inflamed and result in progressive joint degeneration, which increases friction between facets and become a pain generator. The term FJS has been coined to describe condition related to this chain of degeneration and the pain accompanied by it. As facet joint degeneration progresses, bony spurs or synovial cysts can form, which can cause additional pain.
Treatments of facet joints pain include intraarticular injections, medial branch blocks, and RF ablation of targeted nerve root. Facet joint RF denervation for the treatment of facet joint, first described by Shealy in 197520), and is a well established treatment modality the efficacy of which has been proven to provide significant and satisfactory pain relief in patients with CLBP that are refractory to more conservative treatment options3,4,11). Although its efficacy in short term pain relief has been proven in numerous articles, a number of studies suggest that the pain relief is only temporary, lasting only a few months at best13,22). A number of patients experience recurrence of back pain as the medial branch of dorsal ramus regenerates. Moreover, some papers based on cadaveric dissections state that branches of dorsal ramus has multiple variation in its number and location2,18). When these factors are taken into account, successful selective ablation and denervation of the medial branch using traditional RF would not be easily achievable in all of the patients when the medial branch is not confirmed by visualization.
Endoscope guided RF ablation of medial branch has distinctive advantages over traditional RF ablation that high quality visualization of the target branch is possible, which may significantly improve the success rate of ablation. Moreover, while only a point ablation of the target medial branch can be achieved with traditional RF ablation, with endoscope guided RF ablation the operator can denervate multiple spots of target medial branch within the view of the endoscope by employing wanding maneuver of the endoscope and RF bipolar probe. Clear visualization of the operative field enables the surgeon to visually distinguish the target medial branch from other branches of the dorsal ramus, even in cases where there is anatomic variation of the dorsal ramus branches. In accordance with this, our results show both VAS and K-ODI scores improving significantly after the procedure, as well as high patient satisfaction rate.
The endpoint in our procedure was when no further pain was elicited despite provocation of medial branch, even when clear identification of the target medial branch was not achieved. This is important because in more than 2/3 of the cases we could not clearly visualize and identify the medial branch under endoscopic view, partly due to anatomic variations of location and course of medial branch among patients, and partly due to surrounding soft tissue obscuring the view. There were also some cases in which intermediate, lateral and medial branches were not clearly distinguishable from each other. However, instead of trying to exactly pinpoint the medial branch, which may lengthen operation time, we stimulated the area estimated to be medial branch and communicated with the patient to see if any pain was elicited. An area within approximate radius of 3 mm can be denervated without repositioning the endoscope. With this technique, which we termed 'functional visualization', the duration of procedure can be greatly shortened, while effectively denervating patient's pain generators. We also maintained continuous saline irrigation through the endoscope during the entire course of the procedure to avoid thermal damage to the surrounding structures.
When employing this endoscopic method for RF denervation, patient selection and strict adherence to the inclusion criteria is critically important. In majority of cases of CLBP, the source of pain is multifactorial, and include disc, facet joints and sacroiliac joints. Medial branch block alone may not be sufficient to satisfactorily alleviate the patient's symptoms. We excluded patients with discogenic pain, sacroiliac pain and instability as the main source of their symptoms. A thorough physical and neurological examination, as well as appropriate provocation tests such as MBB should be performed beforehand to exclude other possible pain mechanisms.
There are some limitations to our study in its design. Firstly, our study is a retrospective design with a small patient group, and no statistical analysis was done to compare the clinical outcomes of this technique with those of conventional treatment options, including conventional RF technique. Secondly, psychosocial factors which may influence the patient's perception of pain were also not considered. The results of this study represent only a preliminary results of this new technique. We plan to do a prospective randomized study taking into consideration multiple contributing factors to back pain in the future.
CONCLUSION
Endoscopic RF denervation of the medial branch of dorsal ramus significantly improved VAS scores and K-ODI in 52 patients with CLBP for up to 24 months. There were no complications associated with the procedure, and patient satisfaction rate was high. Endoscopic RF denervation of the medial branch could be a safe and effective alternative treatment modality that offers long term pain relief for CLBP of facet origin.
References
- 1.Badgley CE. Pain of spinal origin. J Mich State Med Soc. 1947;46:812. [PubMed] [Google Scholar]
- 2.Bogduk N, Wilson AS, Tynan W. The human lumbar dorsal rami. J Anat. 1982;134(Pt 2):383–397. [PMC free article] [PubMed] [Google Scholar]
- 3.Boswell MV, Colson JD, Sehgal N, Dunbar EE, Epter R. A systematic review of therapeutic facet joint interventions in chronic spinal pain. Pain Physician. 2007;10:229–253. [PubMed] [Google Scholar]
- 4.Boswell MV, Trescot AM, Datta S, Schultz DM, Hansen HC, Abdi S, et al. Interventional techniques : evidence-based practice guidelines in the management of chronic spinal pain. Pain Physician. 2007;10:7–111. [PubMed] [Google Scholar]
- 5.Carragee EJ, Don AS, Hurwitz EL, Cuellar JM, Carrino JA, Herzog R. 2009 ISSLS Prize Winner : Does discography cause accelerated progression of degeneration changes in the lumbar disc : a ten-year matched cohort study. Spine (Phila Pa 1976) 2009;34:2338–2345. doi: 10.1097/BRS.0b013e3181ab5432. [DOI] [PubMed] [Google Scholar]
- 6.Cavanaugh JM, Lu Y, Chen C, Kallakuri S. Pain generation in lumbar and cervical facet joints. J Bone Joint Surg Am. 2006;88(Suppl 2):63–67. doi: 10.2106/JBJS.E.01411. [DOI] [PubMed] [Google Scholar]
- 7.Datta S, Lee M, Falco FJ, Bryce DA, Hayek SM. Systematic assessment of diagnostic accuracy and therapeutic utility of lumbar facet joint interventions. Pain Physician. 2009;12:437–460. [PubMed] [Google Scholar]
- 8.Freburger JK, Holmes GM, Agans RP, Jackman AM, Darter JD, Wallace AS, et al. The rising prevalence of chronic low back pain. Arch Intern Med. 2009;169:251–258. doi: 10.1001/archinternmed.2008.543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Jhala A, Mistry M. Endoscopic lumbar discectomy : experience of first 100 cases. Indian J Orthop. 2010;44:184–190. doi: 10.4103/0019-5413.62051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Katz JN. Lumbar disc disorders and low-back pain : socioeconomic factors and consequences. J Bone Joint Surg Am. 2006;88(Suppl 2):21–24. doi: 10.2106/JBJS.E.01273. [DOI] [PubMed] [Google Scholar]
- 11.Lakemeier S, Lind M, Schultz W, Fuchs-Winkelmann S, Timmesfeld N, Foelsch C, et al. A comparison of intraarticular lumbar facet joint steroid injections and lumbar facet joint radiofrequency denervation in the treatment of low back pain : a randomized, controlled, double-blind trial. Anesth Analg. 2013;117:228–235. doi: 10.1213/ANE.0b013e3182910c4d. [DOI] [PubMed] [Google Scholar]
- 12.Laslett M, Oberg B, Aprill CN, McDonald B. Zygapophysial joint blocks in chronic low back pain : a test of Revel's model as a screening test. BMC Musculoskelet Disord. 2004;5:43. doi: 10.1186/1471-2474-5-43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Leclaire R, Fortin L, Lambert R, Bergeron YM, Rossignol M. Radiofrequency facet joint denervation in the treatment of low back pain : a placebo-controlled clinical trial to assess efficacy. Spine (Phila Pa 1976) 2001;26:1411–1416. doi: 10.1097/00007632-200107010-00003. discussion 1417. [DOI] [PubMed] [Google Scholar]
- 14.Manchikanti L, Boswell MV, Singh V, Benyamin RM, Fellows B, Abdi S, et al. Comprehensive evidence-based guidelines for interventional techniques in the management of chronic spinal pain. Pain Physician. 2009;12:699–802. [PubMed] [Google Scholar]
- 15.Manchikanti L, Pampati V, Fellows B, Bakhit CE. Prevalence of lumbar facet joint pain in chronic low back pain. Pain Physician. 1999;2:59–64. [PubMed] [Google Scholar]
- 16.McLain RF, Pickar JG. Mechanoreceptor endings in human thoracic and lumbar facet joints. Spine (Phila Pa 1976) 1998;23:168–173. doi: 10.1097/00007632-199801150-00004. [DOI] [PubMed] [Google Scholar]
- 17.Poetscher AW, Gentil AF, Lenza M, Ferretti M. Radiofrequency denervation for facet joint low back pain : a systematic review. Spine (Phila Pa 1976) 2014;39:E842–E849. doi: 10.1097/BRS.0000000000000337. [DOI] [PubMed] [Google Scholar]
- 18.Saito T, Steinke H, Miyaki T, Nawa S, Umemoto K, Miyakawa K, et al. Analysis of the posterior ramus of the lumbar spinal nerve : the structure of the posterior ramus of the spinal nerve. Anesthesiology. 2013;118:88–94. doi: 10.1097/ALN.0b013e318272f40a. [DOI] [PubMed] [Google Scholar]
- 19.Schwarzer AC, Derby R, Aprill CN, Fortin J, Kine G, Bogduk N. Pain from the lumbar zygapophysial joints : a test of two models. J Spinal Disord. 1994;7:331–336. [PubMed] [Google Scholar]
- 20.Shealy CN. Facet denervation in the management of back and sciatic pain. Clin Orthop Relat Res. 1976;(115):157–164. [PubMed] [Google Scholar]
- 21.Slipman CW, Bhat AL, Gilchrist RV, Issac Z, Chou L, Lenrow DA. A critical review of the evidence for the use of zygapophysial injections and radiofrequency denervation in the treatment of low back pain. Spine J. 2003;3:310–316. doi: 10.1016/s1529-9430(03)00025-1. [DOI] [PubMed] [Google Scholar]
- 22.van Wijk RM, Geurts JW, Wynne HJ, Hammink E, Buskens E, Lousberg R, et al. Radiofrequency denervation of lumbar facet joints in the treatment of chronic low back pain : a randomized, double-blind, sham lesion-controlled trial. Clin J Pain. 2005;21:335–344. doi: 10.1097/01.ajp.0000120792.69705.c9. [DOI] [PubMed] [Google Scholar]