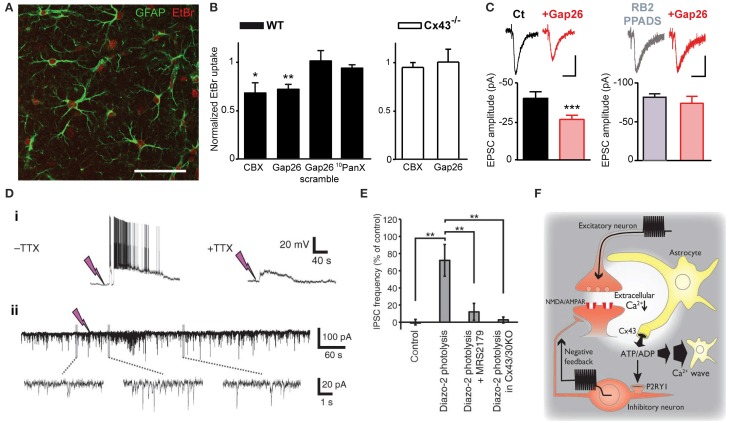
Figure 1.
Astrocytic Cx43 HCs modulate synaptic transmission in hippocampal slices. (A–C) Basal astroglial Cx43 HC activity enhances excitatory synaptic transmission via ATP signaling. (A) Representative image showing ethidium bromide uptake (EtBr; red) in astrocytes (immunostained for GFAP; green) of the stratum radiatum in an acute hippocampal slice. Scale bar, 50 μm. (B) Bar graphs showing astrocytic EtBr uptake in brain slices obtained from wild-type (WT) and astroglial conditional Cx43 KO (Cx43−/−) mice normalized to control (untreated) conditions. Uptake was significantly deceased in WT slices treated with carbenoxolone (CBX, 200 μM) and Gap26 (100 μM), but not Gap26 scramble (100 μM) and 10panx (400 μM) peptides. In Cx43−/− slices, however, both CBX and Gap26 had no significant effect. (C) Bar graph on the left showing a decrease in amplitude of evoked EPSC recorded in CA1 pyramidal neurons during Gap26 application (red) as compared to before (Ct, black). Bar graph on the right showing that pretreatment with ATP P2 receptor antagonists (RB2 + PPADS, gray) occludes the effect of Gap26 (red). Sample traces of corresponding evoked EPSCs are shown above. Scale bar: 20 pA, 20 ms (left); 40 pA, 40 ms (right). (D–F) Cx43 HCs in astrocytes promote feedback inhibitory transmission by releasing ATP. (D) Representative recordings showing that photolysis of diazo-2, represented by lightning bolts, (i) evokes depolarization and bursting in interneurons, with the depolarization persisting with 1 μM TTX, and (ii) transiently increases the frequency of spontaneous inhibitory postsynaptic currents (IPSCs) in CA1 pyramidal neurons. (E) Bar graph indicating increased IPSC frequency in pyramidal neurons after diazo-2 photolysis compared to control condition. This effect was blocked by the P2Y1 receptor antagonist MRS2179 (50 μM) or in brain slices prepared from Cx43/Cx30KO mice. (F) Schematic diagram illustrating a proposed negative feedback mechanism during excitatory transmission. During glutamatergic signaling, Ca2+ influx into neurons results in a localized decrease in [Ca2+]e, which in turn opens Cx43 HCs on astrocytes through which ATP is released. ATP can either trigger slowly propagating astrocytic Ca2+ waves or, when degraded to ADP, depolarize and increase firing in interneurons via P2Y1 receptors, thereby enhancing inhibitory transmission. *p < 0.05; **p < 0.01; ***p < 0.001. Adapted, with permission, from Torres et al. (2012) (D–F).